Deluging Thrombosis: An Unusual Presentation of Acute Promyelocytic Leukemia
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(02): 282-284
DOI: DOI: 10.4103/ijmpo.ijmpo_227_18
Abstract
Acute promyelocytic leukemia (APML) patients are prone to thrombosis. However, thrombosis at presentation is rare in APML. Our patient presented with thrombosis and cytopenia, and the clinical diagnosis was of paroxysmal nocturnal hemoglobinuria. However, subsequent peripheral blood smear and bone marrow study confirmed the diagnosis of APML. The patient was successfully managed with anticoagulation, arsenic trioxide, and all-trans retinoic acid.
Keywords
Acute promyelocytic leukemia - anti-coagulation - chemotherapy - thrombosisPublication History
Received: 17 October 2018
Accepted: 20 June 2019
Article published online:
23 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Acute promyelocytic leukemia (APML) patients are prone to thrombosis. However, thrombosis at presentation is rare in APML. Our patient presented with thrombosis and cytopenia, and the clinical diagnosis was of paroxysmal nocturnal hemoglobinuria. However, subsequent peripheral blood smear and bone marrow study confirmed the diagnosis of APML. The patient was successfully managed with anticoagulation, arsenic trioxide, and all-trans retinoic acid.
Keywords
Acute promyelocytic leukemia - anti-coagulation - chemotherapy - thrombosisIntroduction
Acute promyelocytic leukemia (APML), a unique subtype of acute myeloid leukemia, is commonly associated with a complex life-threatening coagulopathy.[1] Induction therapy with all-trans retinoic acid (ATRA) plus arsenic trioxide (ATO) usually results in a durable remission. As compared to bleeding manifestations, APML-associated thrombotic events are less commonly reported.[2] They can occur either before, during, or after the induction therapy (40.4% prior to induction therapy) and exhibit predilection for both arterial (more common) and venous vasculature.[3] [4] About half of these patients (51.6%) have coagulation defects.[4]
Case Report
A 20-year-old daily wage laborer was admitted with the complaints of the left lower limb swelling for a 2-week duration. Six weeks prior to admission, he was evaluated in a local hospital for low-grade fever and fatigue. Investigations done there had revealed anemia (Hb 72 g/L) and normal chest X-ray; peripheral smear was not examined. He was administered one unit of blood transfusion and discharged on oral haematinics. Six weeks later (now), he was referred to us for worsening of fatigue in addition to left lower limb swelling and dry cough. He complained of significant weight loss for 2 months; past and family history was unremarkable for similar complaints, hemolytic anemia, high-risk behavior, intravenous drug abuse, and tuberculosis. At presentation, he had a fever (100.5°F), tachycardia (112 bpm), and normal BP (114/76 mmHg). Apart from being severely malnourished (body mass index 16.4 kg/m2), he had pallor and left lower limb pitting edema; liver and spleen were not palpable. Doppler ultrasound revealed left lower limb deep vein thrombosis [Figure 1]. Two-dimensional ECHO revealed 2.3 cm × 1.5 cm pedunculated mass adherent to the interventricular septum in the left ventricle [Figure 2] and no features of the right ventricular dysfunction. The left moderate pleural effusion and bilateral pleural-based pulmonary infarcts were evident on computed tomography chest [Figure 3]. He was put on subcutaneous enoxaparin. His hemogram revealed normocytic normochromic anemia (Hb 6.3 g/dL), leukopenia (total leucocyte count [TLC] 1.6 × 109/L), and normal platelet count (334 × 109/L). Work up for paroxysmal nocturnal hemoglobinuria was planned in view of bicytopenia with thrombosis. Meanwhile, 34% blasts and atypical promyelocytes were seen on the peripheral blood smear. Coagulation screen was unremarkable except isolated prolonged prothrombin time (24 s). Presuming the diagnosis of APML, ATRA, and ATO-based induction therapy was started promptly, and two units of packed red blood cells were transfused. Bone marrow examination and flow cytometry were consistent with APML (CD13+, CD33+, HLA-DR−, no aberrant expression markers) [Figure 4]. PML-RARA fusion gene detection by qualitative reverse-transcriptase-polymerase chain reaction (RT-PCR) confirmed the diagnosis of APML (low risk). Blood cultures were sterile; pleural fluid analysis revealed exudative pattern and no malignant cells. Investigative work up for pulmonary tuberculosis and HIV was inconclusive. Fever resolved by day 7 of induction therapy. Hemorrhage, ischemia, and differentiation syndrome were not observed during the induction therapy. The complete hematological response was achieved on day 27. End of induction bone marrow was in remission, and qualitative RT-PCR for PML-RARA was negative. Consolidation chemotherapy was continued along with low molecular weight heparin.
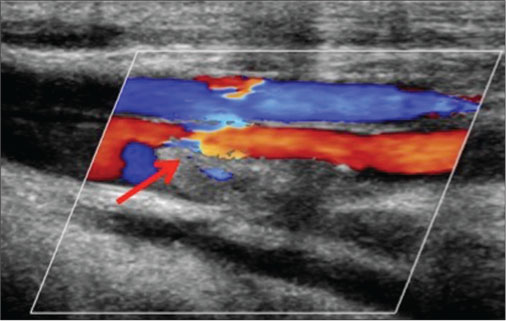
| Figure 1: Doppler ultrasonography showing absence of color flowin the left common femoral vein suggestive of thrombosis
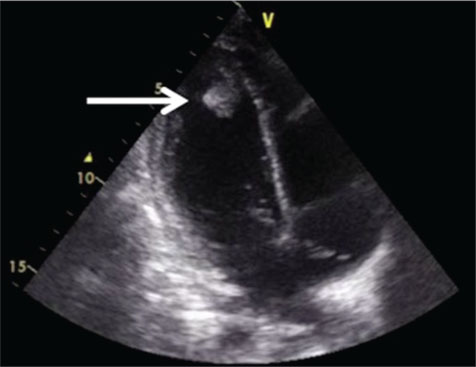
| Figure 2: Two dimensional-Echocardiography showing 2.3 cm × 1.5 cm pedunculated mass within left ventricular cavity adherent to interventricular septum
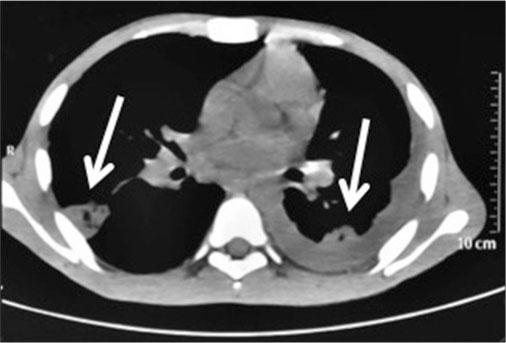
| Figure 3: Computed tomography-chest showing bilateral pleural-based pulmonary infarcts with left-sided pleural effusion
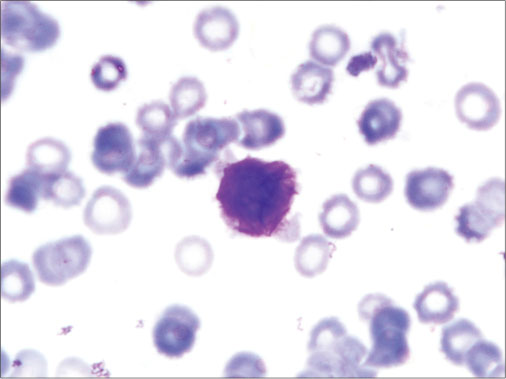
| Figure 4: Bone marrow aspiration slide showing blast with multiple Auer rods (Faggot cells)
Discussion
Pathogenesis of thrombosis in APML is incompletely understood. Apart from procoagulant phenotype of leukemic promyelocytes and concomitant diffuse intravascular coagulation (DIC), ATRA-based therapy per se has also been thought to enhance thrombosis likelihood in such patients.[5] [6] [7] High total leukocyte count at diagnosis (> 10 × 109/L), FLT3 positivity, presence of variant translocation, CD2 or CD15 expression, and occurrence of differentiation syndrome are well-known predisposing factors.[8] [9] The most commonly reported thrombotic complications before APML induction are cerebrovascular accidents, DVT/PE, and cardiac thrombotic events (including myocardial infarction and intraventricular thrombosis).[4] Apart from ATO and ATRA-based therapy, these patients also require anticoagulation for the thrombus.[10] [11] [12]
The index case had an elusive presentation in the form of fever and anemia. Although the nutritional deficiency is the most common cause of anemia in developing countries, meticulous peripheral blood smear examination can pinpoint catastrophic etiologies at an early stage. There was a step-wise clinical progression with lower limb DVT followed by pulmonary embolism, pulmonary infarction, and cardiac involvement. At the time of diagnosis, white blood cell (WBC) count was surprisingly low and other thrombosis predisposing risk factors were conspicuously absent (DIC, FLT3, CD2 or CD15 expression, and differentiation syndrome). The formation of intravascular leukemic thrombi can be offered as an explanation for low initial WBC count, but the same could not be proved by histological examination in the index case. Importantly, neither the existing thromboses worsened nor any new thrombotic events occurred during ATRA plus ATO therapy. Hence, ATRA-based induction therapy can be safely considered in APML patients who have thrombosis prior to induction therapy.
Conclusion
Hematological abnormalities should be thoroughly investigated in patients presenting with thromboembolic manifestations. Thrombosis in APML is an uncommon yet dire complication that can occur even in the absence of known risk features. Successful outcome in APML complicated by thrombosis can be achieved with ATRA plus ATO-based therapy.
Informed consent
Informed signed written consent was taken from the patient involved.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
References
- Falanga A, Barbui T. Coagulopathy of acute promyelocytic leukemia. Acta Haematol 2001; 106: 43-51
- Choudhry A, DeLoughery TG. Bleeding and thrombosis in acute promyelocytic leukemia. Am J Hematol 2012; 87: 596-603
- DiGiovanni Jr. RJ, Crilley P, Kerstein MD. Peripheral arterial occlusion in acute promyelocytic leukemia. Cardiovasc Surg 1999; 7: 258-60
- Rashidi A, Silverberg ML, Conkling PR, Fisher SI. Thrombosis in acute promyelocytic leukemia. Thromb Res 2013; 131: 281-9
- Tallman MS, Lefèbvre P, Baine RM, Shoji M, Cohen I, Green D. et al. Effects of all-trans retinoic acid or chemotherapy on the molecular regulation of systemic blood coagulation and fibrinolysis in patients with acute promyelocytic leukemia. J Thromb Haemost 2004; 2: 1341-50
- Escudier SM, Kantarjian HM, Estey EH. Thrombosis in patients with acute promyelocytic leukemia treated with and without all-trans retinoic acid. Leuk Lymphoma 1996; 20: 435-9
- Dally N, Hoffman R, Haddad N, Sarig G, Rowe JM, Brenner B. Predictive factors of bleeding and thrombosis during induction therapy in acute promyelocytic leukemia-a single center experience in 34 patients. Thromb Res 2005; 116: 109-14
- Chotai PN, Kasangana K, Chandra AB, Rao AS. Recurrent arterial thrombosis as a presenting feature of a variant M3-acute promyelocytic leukemia. Vasc Specialist Int 2016; 32: 65-71
- Jain A, Lad D, Malhotra P, Bommannan K. Acute promyelocytic leukaemia: Looking through ‘gums’. BMJ Case Rep 2016;2016. pii: bcr2016217457.
- Malhotra P, Varma N, Arora N, Das R, Nath A, Patel FD. et al. Treatment of therapy related acute promyelocytic leukemia with the combination of all trans retinoic acid and arsenic trioxide without chemotherapy: A series of three patients. Leuk Lymphoma 2010; 51: 933-6
- Jandial A, Mishra K, Kumar S, Malhotra P. Pulmonary saddle thrombus. QJM 2018; 111: 907-8
- Bhattacharyya D, Rajput AK, Mishra K. Medicine Update. 20. New Delhi: Jaypee Brothers Medical Publishers; 2010: 788-90
Address for correspondence
Publication History
Received: 17 October 2018
Accepted: 20 June 2019
Article published online:
23 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1: Doppler ultrasonography showing absence of color flowin the left common femoral vein suggestive of thrombosis

| Figure 2: Two dimensional-Echocardiography showing 2.3 cm × 1.5 cm pedunculated mass within left ventricular cavity adherent to interventricular septum

| Figure 3: Computed tomography-chest showing bilateral pleural-based pulmonary infarcts with left-sided pleural effusion

| Figure 4: Bone marrow aspiration slide showing blast with multiple Auer rods (Faggot cells)
- 1 Falanga A, Barbui T. Coagulopathy of acute promyelocytic leukemia. Acta Haematol 2001; 106: 43-51
- 2 Choudhry A, DeLoughery TG. Bleeding and thrombosis in acute promyelocytic leukemia. Am J Hematol 2012; 87: 596-603
- 3 DiGiovanni Jr. RJ, Crilley P, Kerstein MD. Peripheral arterial occlusion in acute promyelocytic leukemia. Cardiovasc Surg 1999; 7: 258-60
- 4 Rashidi A, Silverberg ML, Conkling PR, Fisher SI. Thrombosis in acute promyelocytic leukemia. Thromb Res 2013; 131: 281-9
- 5 Tallman MS, Lefèbvre P, Baine RM, Shoji M, Cohen I, Green D. et al. Effects of all-trans retinoic acid or chemotherapy on the molecular regulation of systemic blood coagulation and fibrinolysis in patients with acute promyelocytic leukemia. J Thromb Haemost 2004; 2: 1341-50
- 6 Escudier SM, Kantarjian HM, Estey EH. Thrombosis in patients with acute promyelocytic leukemia treated with and without all-trans retinoic acid. Leuk Lymphoma 1996; 20: 435-9
- 7 Dally N, Hoffman R, Haddad N, Sarig G, Rowe JM, Brenner B. Predictive factors of bleeding and thrombosis during induction therapy in acute promyelocytic leukemia-a single center experience in 34 patients. Thromb Res 2005; 116: 109-14
- 8 Chotai PN, Kasangana K, Chandra AB, Rao AS. Recurrent arterial thrombosis as a presenting feature of a variant M3-acute promyelocytic leukemia. Vasc Specialist Int 2016; 32: 65-71
- 9 Jain A, Lad D, Malhotra P, Bommannan K. Acute promyelocytic leukaemia: Looking through ‘gums’. BMJ Case Rep 2016;2016. pii: bcr2016217457.
- 10 Malhotra P, Varma N, Arora N, Das R, Nath A, Patel FD. et al. Treatment of therapy related acute promyelocytic leukemia with the combination of all trans retinoic acid and arsenic trioxide without chemotherapy: A series of three patients. Leuk Lymphoma 2010; 51: 933-6
- 11 Jandial A, Mishra K, Kumar S, Malhotra P. Pulmonary saddle thrombus. QJM 2018; 111: 907-8
- 12 Bhattacharyya D, Rajput AK, Mishra K. Medicine Update. 20. New Delhi: Jaypee Brothers Medical Publishers; 2010: 788-90


 PDF
PDF  Views
Views  Share
Share

