Dasatinib-Induced Lymphocytosis and Pleural Effusion in a Patient of Chronic Myeloid Leukemia: A Rare Indian Case Report
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(03): 436-438
DOI: DOI: 10.4103/ijmpo.ijmpo_109_19
Sir,
Chronic myeloid leukemia (CML) is a myeloproliferative disorder associated with the presence of Philadelphia chromosome (t
A 60-year-old postmenopausal diabetic female presented with asymptomatic leukocytosis in December 2017. Clinical and ultrasonography examination revealed the presence of mild hepatosplenomegaly, with no other significant finding. The initial total leukocyte count (TLC) was raised to 31,290/μL with hemoglobin (Hb) 13.0 gm/dL and platelet count ~617,000/μL. Peripheral smear showed leukocytosis with left shift and basophilia consistent with clinical suspicion of CML-chronic phase. Bone marrow aspiration and biopsy showed hypercellular marrow with myeloid prominence and basophilia. Reticulin stain did not show any increase in fibrosis. Conventional karyotyping studies revealed abnormal pseudodiploid female karyotype with clonal abnormality and translocation (9:22), (q34:q11.2) in all 20 cells along with BCR-ABL translocation on FISH analysis. Based on investigational findings, the female was diagnosed with CML. The patient was started on treatment with oral dasatinib tablets in January 2018, 100 mg OD for 3 months.
The patient underwent complete hematological response in 1½ months after dasatinib initiation, and treatment was continued subsequently. The initial RQ-PCR BCR-ABL levels were obtained (~21.28%) in February 2018, with follow-up RQ-PCR BCR ABL1 IS in May 2018 (~0.0364%). In June 2018, follow-up CBC evaluation showed a drop in Hb to 9.7 gm/dL with TLC of 14,210/μL and platelet counts of 129,000/μL. WBC morphology had absolute lymphocytosis of ~7815 cells/μL with the presence of LGL. A follow-up RQ-PCR BCR ABL1 IS in August 2018 was ~0.0126% (almost approaching the MMR-4 cutoff of 0.01%). Absolute lymphocytosis was found to be persistent till December 2018. At this follow-up, the patient complained of fatigue and mild breathlessness along with low-grade fever. LDH, ferritin levels, iron studies, and Vitamin B12 levels were within normal limits. RQ-PCR BCR ABL1 IS at this time was ~0.0241%. Although the patient improved symptomatically, the complete blood count (CBC) follow-up showed further drop in Hb to 7.9 gm/dL, TLC levels of 12,710 cells/μL with 56%-lymphocytes and platelet count ~142,000/μL. Bone marrow examination and flow cytometry ruled out lymphoid clonality. Chest X-ray revealed the presence of unilateral pleural effusion. At this time point, dose of dasatinib was reduced to 50 mg OD. The patient was started on steroid treatment (tablet prednisolone 1 mg/kg OD for 2 weeks) for the management of pleural effusion. Follow-up CBC evaluation after a gap of 12 days showed Hb 8.3 gm/dL and TLC ~13,410 cells/μL with ~32%-lymphocytes and platelet count ~3,04,000/μL. Bone marrow aspirate and biopsy evaluation revealed cellular marrow with trilineage hematopoiesis. Immunophenotyping was done on peripheral blood with flow cytometry using panel of multiple antibodies in view of persistent absolute lymphocytosis [Figure 1]. Of the gated lymphocytes ~32%, the T-cells ~42%-showed normal patterns of maturation, B-cells ~5%-were polyclonal on kappa and lambda light chain immunostaining, while an increase in NK cells ~40% (absolute count ~1716/μl) was seen showing normal CD7 expression, while half of the NK cell population showed dim to negative CD56 expression.
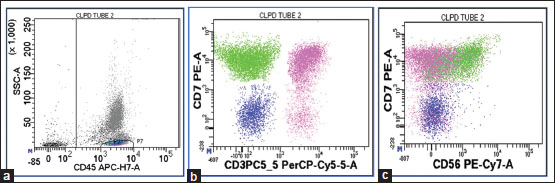
| Figure 1:Flow cytometric dot pots – (a) Lymphocytes gated on CD45-SSC dot plot. (b) Pink-colored population is T-cells, Green-colored population is NK cells, and Blue-colored population is B cells. (c) NK cell population is seen showing dim to negative CD56 expression
Large granular lymphocytosis is defined as (1) an absolute lymphocyte count >3.000–3.600 × 109/l and absolute LGL count >1.500 × 109/l and (2) predominance of LGLs in the peripheral blood smears on at least one occasion during dasatinib treatment.[4] Based on the current CBC, the absolute NK cell number was less. On repeating the CBC in January 2019, the Hb was better though still below normal range (9.6 g/dL) and WBC count ~23,200/μL with 80%- neutrophils and platelet count ~197,000/μL.
After stopping of dasatinib and initiation of steroid therapy, pleural effusion subsided as confirmed on chest X-ray. Prednisolone was tapered gradually after 2 weeks to 0.5 mg/kg OD for 1 week and 0.2 mg/kg over the next week and then it was stopped. The last follow-up CBC counts were relatively stable and showed Hb ~11.0 gm/dL and TLC ~13,650 cells/μL, with ~85%-neutrophils and platelet count ~219,000/μL. Oral dasatinib was then restarted at a lower dose (50 mg OD) after the lymphocytes were confirmed to be within normal limits and pleural effusion was cured.
Most of the pleural effusions following dasatinib therapy are exudative and may require thoracocentesis.[5] However, the patient in this case required only steroid therapy and dasatinib discontinuation for the subsidence of pleural effusion. In this case, lymphocytosis preceded the occurrence of pleural effusion, which reiterated the fact present in scientific literature that dasatinib-related pleural effusion occurs as a result of LGL proliferation.[6] In fact, there is evidence that lymphocytosis and consequent pleural effusion following dasatinib therapy are associated with improved therapeutic outcome. This statement can be somewhat supported by this case as the bone marrow studies revealed normal trilinear hematopoiesis 3 months after dasatinib therapy initiation.
It has been speculated that SRC group of kinases, which have a crucial role in lymphocyte trafficking between intravascular regions and tissues, are inhibited by dasatinib which may be the reason for lymphocytic alterations.[7] There are no clear-cut guidelines for the treatment of pleural effusion, which occurs due to dasatinib. Published literature mentions the discontinuation of dasatinib and starting of steroid or diuretic therapy for resolving pleural effusion.[8] There is evidence that continuing dasatinib therapy again with a lower therapeutic dose can help prevent adverse effects.[9]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Acknowledgement
The authors would like to thank Biomic Life Science, a Clinical Research Organization, for providing assistance in manuscript writing.
Publication History
Received: 01 May 2019
Accepted: 20 October 2019
Article published online:
28 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Sir,
Chronic myeloid leukemia (CML) is a myeloproliferative disorder associated with the presence of
Philadelphia chromosome (t
A 60-year-old postmenopausal diabetic female presented with asymptomatic leukocytosis in December 2017. Clinical and ultrasonography examination revealed the presence of mild hepatosplenomegaly, with no other significant finding. The initial total leukocyte count (TLC) was raised to 31,290/μL with hemoglobin (Hb) 13.0 gm/dL and platelet count ~617,000/μL. Peripheral smear showed leukocytosis with left shift and basophilia consistent with clinical suspicion of CML-chronic phase. Bone marrow aspiration and biopsy showed hypercellular marrow with myeloid prominence and basophilia. Reticulin stain did not show any increase in fibrosis. Conventional karyotyping studies revealed abnormal pseudodiploid female karyotype with clonal abnormality and translocation (9:22), (q34:q11.2) in all 20 cells along with BCR-ABL translocation on FISH analysis. Based on investigational findings, the female was diagnosed with CML. The patient was started on treatment with oral dasatinib tablets in January 2018, 100 mg OD for 3 months.
The patient underwent complete hematological response in 1½ months after dasatinib initiation, and treatment was continued subsequently. The initial RQ-PCR BCR-ABL levels were obtained (~21.28%) in February 2018, with follow-up RQ-PCR BCR ABL1 IS in May 2018 (~0.0364%). In June 2018, follow-up CBC evaluation showed a drop in Hb to 9.7 gm/dL with TLC of 14,210/μL and platelet counts of 129,000/μL. WBC morphology had absolute lymphocytosis of ~7815 cells/μL with the presence of LGL. A follow-up RQ-PCR BCR ABL1 IS in August 2018 was ~0.0126% (almost approaching the MMR-4 cutoff of 0.01%). Absolute lymphocytosis was found to be persistent till December 2018. At this follow-up, the patient complained of fatigue and mild breathlessness along with low-grade fever. LDH, ferritin levels, iron studies, and Vitamin B12 levels were within normal limits. RQ-PCR BCR ABL1 IS at this time was ~0.0241%. Although the patient improved symptomatically, the complete blood count (CBC) follow-up showed further drop in Hb to 7.9 gm/dL, TLC levels of 12,710 cells/μL with 56%-lymphocytes and platelet count ~142,000/μL. Bone marrow examination and flow cytometry ruled out lymphoid clonality. Chest X-ray revealed the presence of unilateral pleural effusion. At this time point, dose of dasatinib was reduced to 50 mg OD. The patient was started on steroid treatment (tablet prednisolone 1 mg/kg OD for 2 weeks) for the management of pleural effusion. Follow-up CBC evaluation after a gap of 12 days showed Hb 8.3 gm/dL and TLC ~13,410 cells/μL with ~32%-lymphocytes and platelet count ~3,04,000/μL. Bone marrow aspirate and biopsy evaluation revealed cellular marrow with trilineage hematopoiesis. Immunophenotyping was done on peripheral blood with flow cytometry using panel of multiple antibodies in view of persistent absolute lymphocytosis [Figure 1]. Of the gated lymphocytes ~32%, the T-cells ~42%-showed normal patterns of maturation, B-cells ~5%-were polyclonal on kappa and lambda light chain immunostaining, while an increase in NK cells ~40% (absolute count ~1716/μl) was seen showing normal CD7 expression, while half of the NK cell population showed dim to negative CD56 expression.

| Figure 1:Flow cytometric dot pots – (a) Lymphocytes gated on CD45-SSC dot plot. (b) Pink-colored population is T-cells, Green-colored population is NK cells, and Blue-colored population is B cells. (c) NK cell population is seen showing dim to negative CD56 expression
Large granular lymphocytosis is defined as (1) an absolute lymphocyte count >3.000–3.600 × 109/l and absolute LGL count >1.500 × 109/l and (2) predominance of LGLs in the peripheral blood smears on at least one occasion during dasatinib treatment.[4] Based on the current CBC, the absolute NK cell number was less. On repeating the CBC in January 2019, the Hb was better though still below normal range (9.6 g/dL) and WBC count ~23,200/μL with 80%- neutrophils and platelet count ~197,000/μL.
After stopping of dasatinib and initiation of steroid therapy, pleural effusion subsided as confirmed on chest X-ray. Prednisolone was tapered gradually after 2 weeks to 0.5 mg/kg OD for 1 week and 0.2 mg/kg over the next week and then it was stopped. The last follow-up CBC counts were relatively stable and showed Hb ~11.0 gm/dL and TLC ~13,650 cells/μL, with ~85%-neutrophils and platelet count ~219,000/μL. Oral dasatinib was then restarted at a lower dose (50 mg OD) after the lymphocytes were confirmed to be within normal limits and pleural effusion was cured.
Most of the pleural effusions following dasatinib therapy are exudative and may require thoracocentesis.[5] However, the patient in this case required only steroid therapy and dasatinib discontinuation for the subsidence of pleural effusion. In this case, lymphocytosis preceded the occurrence of pleural effusion, which reiterated the fact present in scientific literature that dasatinib-related pleural effusion occurs as a result of LGL proliferation.[6] In fact, there is evidence that lymphocytosis and consequent pleural effusion following dasatinib therapy are associated with improved therapeutic outcome. This statement can be somewhat supported by this case as the bone marrow studies revealed normal trilinear hematopoiesis 3 months after dasatinib therapy initiation.
It has been speculated that SRC group of kinases, which have a crucial role in lymphocyte trafficking between intravascular regions and tissues, are inhibited by dasatinib which may be the reason for lymphocytic alterations.[7] There are no clear-cut guidelines for the treatment of pleural effusion, which occurs due to dasatinib. Published literature mentions the discontinuation of dasatinib and starting of steroid or diuretic therapy for resolving pleural effusion.[8] There is evidence that continuing dasatinib therapy again with a lower therapeutic dose can help prevent adverse effects.[9]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Acknowledgement
The authors would like to thank Biomic Life Science, a Clinical Research Organization, for providing assistance in manuscript writing.
Conflict of Interest
There are no conflicts of interest.
References
- Ghiuzeli CM. Dasatinib-associated transient peripheral blood appearance of a clonal large granular lymphocytic population in a Philadelphia-positive acute lymphoblastic leukemia patient: A case report. Rom J Oncol Hematol 2014; 2: 30-4
- Bergeron A, Réa D, Levy V, Picard C, Meignin V, Tamburini J. et al. Lung abnormalities after dasatinib treatment for chronic myeloid leukemia: A case series. Am J Respir Crit Care Med 2007; 176: 814-8
- de Lavallade H, Punnialingam S, Milojkovic D, Bua M, Khorashad JS, Gabriel IH. et al. Pleural effusions in patients with chronic myeloid leukaemia treated with dasatinib may have an immune-mediated pathogenesis. Br J Haematol 2008; 141: 745-7
- Paydas S. Dasatinib, large granular lymphocytosis, and pleural effusion: Useful or adverse effect?. Crit Rev Oncol Hematol 2014; 89: 242-7
- Malkan UY, Haznedaroglu IC. A CML case with resistant pleural effusion with tyrosine kinase inhibitor treatment. Int J Clin Exp Med 2016; 9: 14884-7
- Apperley JF, Cortes JE, Kim DW, Roy L, Roboz GJ, Rosti G. et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: The START a trial. J Clin Oncol 2009; 27: 3472-9
- Imagawa J, Tanaka H, Matsumoto K, Morita K, Harada Y, Harada H. Asharp fluctuation in peripheral blood cells shortly after dasatinib administration. Int J Hematol 2012; 96: 194-9
- Ferreiro L, San-José E, Suárez-Antelo J, Valdés L. Dasatinib-induced pleural effusion: Chylothorax, an option to consider. Ann Thorac Med 2016; 11: 289-93
- Cortes J, Kim DW, Raffoux E, Martinelli G, Ritchie E, Roy L. et al. Efficacy and safety of dasatinib in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blast phase. Leukemia 2008; 22: 2176-83
Address for correspondence
Publication History
Received: 01 May 2019
Accepted: 20 October 2019
Article published online:
28
June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301
UP, India

| Figure 1:Flow cytometric dot pots – (a) Lymphocytes gated on CD45-SSC dot plot. (b) Pink-colored population is T-cells, Green-colored population is NK cells, and Blue-colored population is B cells. (c) NK cell population is seen showing dim to negative CD56 expression
References
- Ghiuzeli CM. Dasatinib-associated transient peripheral blood appearance of a clonal large granular lymphocytic population in a Philadelphia-positive acute lymphoblastic leukemia patient: A case report. Rom J Oncol Hematol 2014; 2: 30-4
- Bergeron A, Réa D, Levy V, Picard C, Meignin V, Tamburini J. et al. Lung abnormalities after dasatinib treatment for chronic myeloid leukemia: A case series. Am J Respir Crit Care Med 2007; 176: 814-8
- de Lavallade H, Punnialingam S, Milojkovic D, Bua M, Khorashad JS, Gabriel IH. et al. Pleural effusions in patients with chronic myeloid leukaemia treated with dasatinib may have an immune-mediated pathogenesis. Br J Haematol 2008; 141: 745-7
- Paydas S. Dasatinib, large granular lymphocytosis, and pleural effusion: Useful or adverse effect?. Crit Rev Oncol Hematol 2014; 89: 242-7
- Malkan UY, Haznedaroglu IC. A CML case with resistant pleural effusion with tyrosine kinase inhibitor treatment. Int J Clin Exp Med 2016; 9: 14884-7
- Apperley JF, Cortes JE, Kim DW, Roy L, Roboz GJ, Rosti G. et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: The START a trial. J Clin Oncol 2009; 27: 3472-9
- Imagawa J, Tanaka H, Matsumoto K, Morita K, Harada Y, Harada H. Asharp fluctuation in peripheral blood cells shortly after dasatinib administration. Int J Hematol 2012; 96: 194-9
- Ferreiro L, San-José E, Suárez-Antelo J, Valdés L. Dasatinib-induced pleural effusion: Chylothorax, an option to consider. Ann Thorac Med 2016; 11: 289-93
- Cortes J, Kim DW, Raffoux E, Martinelli G, Ritchie E, Roy L. et al. Efficacy and safety of dasatinib in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blast phase. Leukemia 2008; 22: 2176-83


 PDF
PDF  Views
Views  Share
Share

