Current status of hematopoietic stem cell transplant in chronic myeloid leukemia
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(03): 207-210
DOI: DOI: 10.4103/0971-5851.142036
Abstract
Indications for hematopoietic stem cell transplant (HSCT) in chronic myeloid leukemia (CML) have changed over time. This change has largely been influenced by the advent of tyrosine kinase inhibitors, increased understanding of the mechanisms underlying disease phase progression as well as drug resistance, refinement of transplant techniques and exploitation of graft versus leukemia effect in this disease. Here, we have discussed the status of HSCT in CML in the present era with regards to the current indications, factors determining outcome and management strategies for posttransplant relapse.
Publication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Indications for hematopoietic stem cell transplant (HSCT) in chronic myeloid leukemia (CML) have changed over time. This change has largely been influenced by the advent of tyrosine kinase inhibitors, increased understanding of the mechanisms underlying disease phase progression as well as drug resistance, refinement of transplant techniques and exploitation of graft versus leukemia effect in this disease. Here, we have discussed the status of HSCT in CML in the present era with regards to the current indications, factors determining outcome and management strategies for posttransplant relapse.
INTRODUCTION
Over last three decades, the role of hematopoietic stem cell transplant (HSCT) in chronic myeloid leukemia (CML) has seen several phases and continues to be defined. In 1980's, HSCT came as a revolutionary concept that offered cure from CML.[1] In 1990's, transplant methodologies were refined, which resulted in increased cure rates and decreased transplant-related mortality. Survival outcomes improved significantly in chronic phase patients with 3 years survival rate of more than 80%.[2] Allogeneic HSCT, thus became treatment of choice in CML. Thirty years later, it is still the only treatment that offers cure in CML. The ability of allogeneic HSCT to cure CML is related to the antileukemic effects of both the conditioning regimen and the graft versus leukemia (GVL) effect of the donor lymphocytes. However, with the advent of imatinib in early 2000's the natural history of CML was changed and so did the way we treated CML.[3] With the excellent response rates and minimal toxicity imatinib soon became the treatment of choice for chronic phase CML, and it was thought that HSCT was no longer needed at least in chronic phase.[4,5] However, as our experience with imatinib increased, issues of resistance/intolerance in chronic phase emerged. At 18 months of treatment, imatinib resistance was seen in 15% of chronic phase patients.[6] Also, the treatment of advanced phase disease (accelerated and blast phases) with imatinib proved to be inadequate.[7] Second-generation tyrosine kinase inhibitors (TKIs) including dasatinib and nilotinib were able to achieve <50 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4202616/#ref8" rid="ref8" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_409045469" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>8] Thus, transplantation still has a definite, though more limited, role in the treatment of CML.
CURRENT INDICATIONS OF HEMATOPOIETIC STEM CELL TRANSPLANT IN CHRONIC MYELOID LEUKEMIA
In advanced phase disease, treatment with TKIs can achieve initial good response (second chronic phase). However, such responses are usually short lived and do not appear to be associated with long-term survival.[9,10] In one study, the rate of major CyR and CCyR in accelerated phase patients treated with imatinib at 600 mg/day was only 24% and 17%, respectively.[9] In another analysis of three phase II studies of CML patients in blast crisis treated with 600 mg/day imatinib, at 36 months only 7% were disease free and only 14% were alive.[11] Allogeneic HSCT is the treatment of choice in such cases. However, it would be prudent to start the patient on a TKI for two reasons. First, it tides over the time period required to find human leukocyte antigen (HLA) matched unrelated donor if HLA-matched sibling is not available. Second, cure rates in advanced phase disease are better if transplanted in second chronic phase.[12]
Among patients in chronic phase treated with imatinib as first line TKI therapy, approximately 20% will fail primary therapy, either from intolerance, relapse or progression to advanced phase disease.[6,13] It is reasonable to consider HSCT at the time of first-line failure. However, secondary TKI therapy should be started at the time of resistance for two reasons-first, a CCyR on secondary therapy is achieved in approximately 50% patients[14] second, a donor search may take months. Patients who relapse with a T315I mutation should proceed to transplant at the earliest, given the low-response rate to secondary therapy. Similarly, those who have a mutation within the P loop region, which is known to be associated with an increased risk of progression to advance phase disease, transplant is a reasonable option.
Thus, current indications for transplant in CML include [Figure 1]:
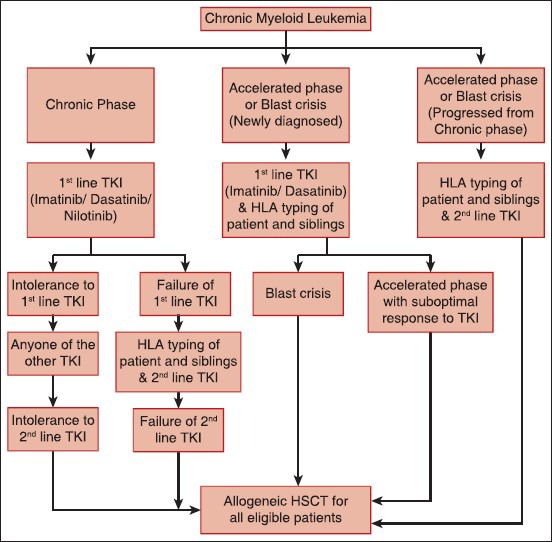
| Figure 1:Algorithm for consideration of hematopoietic stem cell transplant in chronic myeloid leukemia
- Advanced phase disease (accelerated phase and blast crisis)
- Resistance/intolerance to TKIs
- Relapse on TKIs.
FACTORS DETERMINING OUTCOME FOLLOWING ALLOGENEIC HEMATOPOIETIC STEM CELL TRANSPLANT IN CHRONIC MYELOID LEUKEMIA
Several factors influence the outcome of allogeneic HSCT in CML, most importantly the phase of the disease,[15,16] but also, the type of donor used,[17,18] the nature of the stem cell product,[19,20,21] and the age of the patient.[1,22] The European Group for Blood and Marrow Transplantation (EBMT) devised a risk score based on five separate characteristics, which predicted treatment-related mortality and 5 years overall survival following allogeneic hematopoietic cell transplantation.[23] This system uses HLA matching, stage, age, sex of donor/recipient and time from diagnosis to transplant as risk factors. Survival at 5 years varied from 72% for the best score to 22% for the worst score. The EBMT risk score was later validated using separate data from the International Bone Marrow Transplant Registry.[24] However, the value of this prognostic scoring system is largely for prognostication rather than decision-making.
As mentioned, the most important prognostic factor for survival posttransplant is disease phase. Five years disease-free survival varies from 90% in chronic phase to 40-50% in accelerated phase to 10-20% in blast crisis.[15,16] Outcome of patients in blast crisis who achieve a second chronic phase with TKI therapy is similar to that of patients in accelerated phase. Advances in HLA typing and graft versus host disease (GVHD) therapy has improved transplant outcomes in CML.[17] Results with a fully matched unrelated donor are quite similar to that achieved with a matched-related donor.[18] Bone marrow harvest has been replaced by peripheral blood mobilization for stem cell collection making the procedure more donor compliant. Two randomized trials involving patients with various hematologic malignancies, including CML, concluded that use of granulocyte colony-stimulating factor mobilized peripheral blood stem cells lead to faster myeloid and platelet recovery, no significant difference in acute or chronic GVHD and an overall survival advantage compared with bone marrow.[19,20] However, another study in chronic phase CML showed no significant difference in the outcome between the bone marrow and peripheral blood groups.[21] In earlier studies, younger age has been shown to improve survival after HSCT in CML,[1] however, better supportive care, better donor selection and use of reduced intensity conditioning regimens have dampened the effect of age on survival outcome.[22] Some studies had suggested that increased interval from diagnosis to transplant was associated with a worse transplant outcome, higher relapse rate and an increase in nonrelapse mortality.[17,25] This was attributed to the development of resistant clones associated with a longer delay to transplantation. Another factor implicated in affecting outcome in earlier studies was the use of prior imatinib, which was shown to be associated with an increase in regimen-related toxicity and mortality, especially from hepatic causes.[26] However, larger studies have shown that there is no deleterious effect of the pretransplant imatinib on outcome.[27] Furthermore, patients with Abl mutations have similar outcomes compared with those with no mutations following transplantation.
POST-TRANSPLANT RELAPSE
On average 5-20% of the chronic phase patients and 30-60% of advanced phase patients relapse following allo-HSCT.[28] Increased incidence of relapse is seen with HSCT strategies involving T-cell depletion and use of reduced intensity conditioning regimens.[29] Careful monitoring of minimal residual disease should be done to prevent disease relapse posttransplant. An increasing level of BCR-Abl transcript measured by quantitative polymerase chain reaction is predictive of impending disease relapse and needs intervention. Modalities available to prevent and/or treat posttransplant relapse include donor lymphocyte infusion (DLI) therapy, TKI therapy, interferon therapy and infusion of in vitro stimulated CD8+ T cells.[30,31,32] DLI have been very effective (70-90% complete remission rate) for patients who relapse with chronic phase.[33,34] The efficacy of DLI is the result of GVL effect which is highly pronounced in CML. However, the drawbacks of DLI are (1) advanced phases of the disease are least responsive to DLIs, (2) effect of DLI may last for few months and repeat DLI may be required and (3) it may be complicated by life-threatening GVHD. TKI therapy with imatinib is highly effective in treatment of posttransplant relapse.[28,30] In a study of 28 CML patients who were treated with imatinib for posttransplant relapse, 74% and 35% patients achieved complete hematological remission and CCyR, respectively.[28] Longer follow-up of similar patients had shown that these responses are quite durable.[35] Response rates are higher in patients who relapse in chronic phase compared to those who relapse in advanced phase disease.[28] Furthermore, there is some evidence that imatinb maintenance therapy posttransplant is effective in preventing relapse in high-risk cases.[36]
CONCLUSION
Allogeneic HSCT remains the only curative treatment for CML. However, considering the morbidity and mortality associated with HSCT and efficacy and favorable toxicity profile of TKIs, CML chronic phase patients should be initially treated with TKI. For patients with advanced phase disease and those with chronic phase disease who are resistant or intolerant to TKI or relapse on TKI, HSCT is the treatment of choice.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Thomas ED, Clift RA, Fefer A, Appelbaum FR, Beatty P, Bensinger WI, et al. Marrow transplantation for the treatment of chronic myelogenous leukemia. Ann Intern Med 1986;104:155-63.
- Radich JP, Gooley T, Bensinger W, Chauncey T, Clift R, Flowers M, et al. HLA-matched related hematopoietic cell transplantation for chronic-phase CML using a targeted busulfan and cyclophosphamide preparative regimen. Blood 2003;102:31-5.
- O′Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003;348:994-1004.
- Druker BJ, Guilhot F, O′Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006;355:2408-17.
- Hochhaus A, O′Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia 2009;23:1054-61.
- O′Dwyer ME, Mauro MJ, Blasdel C, Farnsworth M, Kurilik G, Hsieh YC, et al. Clonal evolution and lack of cytogenetic response are adverse prognostic factors for hematologic relapse of chronic phase CML patients treated with imatinib mesylate. Blood 2004;103:451-5.
- Oki Y, Kantarjian HM, Gharibyan V, Jones D, O′brien S, Verstovsek S, et al. Phase II study of low-dose decitabine in combination with imatinib mesylate in patients with accelerated or myeloid blastic phase of chronic myelogenous leukemia. Cancer 2007;109:899-906.
- Kantarjian H, O′Brien S, Talpaz M, Borthakur G, Ravandi F, Faderl S, et al. Outcome of patients with Philadelphia chromosome-positive chronic myelogenous leukemia post-imatinib mesylate failure. Cancer 2007;109:1556-60.
- Talpaz M, Silver RT, Druker BJ, Goldman JM, Gambacorti-Passerini C, Guilhot F, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: Results of a phase 2 study. Blood 2002;99:1928-37.
- Kantarjian H, Talpaz M, O′Brien S, Giles F, Faderl S, Verstovsek S, et al. Survival benefit with imatinib mesylate therapy in patients with accelerated-phase chronic myelogenous leukemia - comparison with historic experience. Cancer 2005;103:2099-108.
- Silver RT, Talpaz M, Sawyers CL, Druker BJ, Hocchaus A, Schiffer CA, et al. Four years of follow-up of 1027 patients with late chronic phase (L-CP), accelerated phase (AP), or blast crisis (BC) chronic myeloid leukemia (CML) treated with imatinib in three large phase II trials. Blood 2004;104:Abstract #23.
- Visani G, Rosti G, Bandini G, Tosi P, Isidori A, Malagola M, et al. Second chronic phase before transplantation is crucial for improving survival of blastic phase chronic myeloid leukaemia. Br J Haematol 2000;109:722-8.
- Soverini S, Martinelli G, Rosti G, Bassi S, Amabile M, Poerio A, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: A study by the GIMEMA working party on chronic myeloid leukemia. J Clin Oncol 2005;23:4100-9.
- Milojkovic D, Apperley JF, Gerrard G, Ibrahim AR, Szydlo R, Bua M, et al. Responses to second-line tyrosine kinase inhibitors are durable: An intention-to-treat analysis in chronic myeloid leukemia patients. Blood 2012;119:1838-43.
- Biggs JC, Szer J, Crilley P, Atkinson K, Downs K, Dodds A, et al. Treatment of chronic myeloid leukemia with allogeneic bone marrow transplantation after preparation with BuCy2. Blood 1992;80:1352-7.
- Clift RA, Buckner CD, Thomas ED, Bryant E, Anasetti C, Bensinger WI, et al. Marrow transplantation for patients in accelerated phase of chronic myeloid leukemia. Blood 1994;84:4368-73.
- Enright H, Daniels K, Arthur DC, Dusenbery KE, Kersey JH, Kim T, et al. Related donor marrow transplant for chronic myeloid leukemia: Patient characteristics predictive of outcome. Bone Marrow Transplant 1996;17:537-42.
- Hansen JA, Gooley TA, Martin PJ, Appelbaum F, Chauncey TR, Clift RA, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med 1998;338:962-8.
- Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med 2001;344:175-81.
- Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood 2002;100:1525-31.
- Oehler VG, Radich JP, Storer B, Blume KG, Chauncey T, Clift R, et al. Randomized trial of allogeneic related bone marrow transplantation versus peripheral blood stem cell transplantation for chronic myeloid leukemia. Biol Blood Marrow Transplant 2005;11:85-92.
- Clift RA, Appelbaum FR, Thomas ED. Treatment of chronic myeloid leukemia by marrow transplantation. Blood 1993;82:1954-6.
- Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic leukemia working party of the European group for blood and marrow transplantation. Lancet 1998;352:1087-92.
- Passweg JR, Walker I, Sobocinski KA, Klein JP, Horowitz MM, Giralt SA, et al. Validation and extension of the EBMT Risk Score for patients with chronic myeloid leukaemia (CML) receiving allogeneic haematopoietic stem cell transplants. Br J Haematol 2004;125:613-20.
- Goldman JM, Szydlo R, Horowitz MM, Gale RP, Ash RC, Atkinson K, et al. Choice of pretransplant treatment and timing of transplants for chronic myelogenous leukemia in chronic phase. Blood 1993;82:2235-8.
- Shimoni A, Kröger N, Zander AR, Rowe JM, Hardan I, Avigdor A, et al. Imatinib mesylate (STI571) in preparation for allogeneic hematopoietic stem cell transplantation and donor lymphocyte infusions in patients with Philadelphia-positive acute leukemias. Leukemia 2003;17:290-7.
- Oehler VG, Gooley T, Snyder DS, Johnston L, Lin A, Cummings CC, et al. The effects of imatinib mesylate treatment before allogeneic transplantation for chronic myeloid leukemia. Blood 2007;109:1782-9.
- Kantarjian HM, O′Brien S, Cortes JE, Giralt SA, Rios MB, Shan J, et al. Imatinib mesylate therapy for relapse after allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood 2002;100:1590-5.
- Eapen M, Giralt SA, Horowitz MM, Klein JP, Wagner JE, Zhang MJ, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant 2004;34:721-7.
- Kröger N. Approaches to relapse after allogeneic stem cell transplantation. Curr Opin Oncol 2011;23:203-8.
- Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995;86:2041-50.
- Bornhäuser M, Thiede C, Platzbecker U, Kiani A, Oelschlaegel U, Babatz J, et al. Prophylactic transfer of BCR-ABL-, PR1-, and WT1-reactive donor T cells after T cell-depleted allogeneic hematopoietic cell transplantation in patients with chronic myeloid leukemia. Blood 2011;117:7174-84.
- Kolb HJ, Schmid C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood 2004;103:767-76.
- Dazzi F, Szydlo RM, Craddock C, Cross NC, Kaeda J, Chase A, et al. Comparison of single-dose and escalating-dose regimens of donor lymphocyte infusion for relapse after allografting for chronic myeloid leukemia. Blood 2000;95:67-71.
- DeAngelo DJ, Hochberg EP, Alyea EP, Longtine J, Lee S, Galinsky I, et al. Extended follow-up of patients treated with imatinib mesylate (gleevec) for chronic myelogenous leukemia relapse after allogeneic transplantation: durable cytogenetic remission and conversion to complete donor chimerism without graft-versus-host disease. Clin Cancer Res 2004;10:5065-71.
- Olavarria E, Siddique S, Griffiths MJ, Avery S, Byrne JL, Piper KP, et al. Post transplantation imatinib as a strategy to postpone the requirement for immunotherapy in patients undergoing reduced-intensity allografts for chronic myeloid leukemia. Blood 2007;110:4614-7.

| Figure 1:Algorithm for consideration of hematopoietic stem cell transplant in chronic myeloid leukemia
References
- Thomas ED, Clift RA, Fefer A, Appelbaum FR, Beatty P, Bensinger WI, et al. Marrow transplantation for the treatment of chronic myelogenous leukemia. Ann Intern Med 1986;104:155-63.
- Radich JP, Gooley T, Bensinger W, Chauncey T, Clift R, Flowers M, et al. HLA-matched related hematopoietic cell transplantation for chronic-phase CML using a targeted busulfan and cyclophosphamide preparative regimen. Blood 2003;102:31-5.
- O′Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003;348:994-1004.
- Druker BJ, Guilhot F, O′Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006;355:2408-17.
- Hochhaus A, O′Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia 2009;23:1054-61.
- O′Dwyer ME, Mauro MJ, Blasdel C, Farnsworth M, Kurilik G, Hsieh YC, et al. Clonal evolution and lack of cytogenetic response are adverse prognostic factors for hematologic relapse of chronic phase CML patients treated with imatinib mesylate. Blood 2004;103:451-5.
- Oki Y, Kantarjian HM, Gharibyan V, Jones D, O′brien S, Verstovsek S, et al. Phase II study of low-dose decitabine in combination with imatinib mesylate in patients with accelerated or myeloid blastic phase of chronic myelogenous leukemia. Cancer 2007;109:899-906.
- Kantarjian H, O′Brien S, Talpaz M, Borthakur G, Ravandi F, Faderl S, et al. Outcome of patients with Philadelphia chromosome-positive chronic myelogenous leukemia post-imatinib mesylate failure. Cancer 2007;109:1556-60.
- Talpaz M, Silver RT, Druker BJ, Goldman JM, Gambacorti-Passerini C, Guilhot F, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: Results of a phase 2 study. Blood 2002;99:1928-37.
- Kantarjian H, Talpaz M, O′Brien S, Giles F, Faderl S, Verstovsek S, et al. Survival benefit with imatinib mesylate therapy in patients with accelerated-phase chronic myelogenous leukemia - comparison with historic experience. Cancer 2005;103:2099-108.
- Silver RT, Talpaz M, Sawyers CL, Druker BJ, Hocchaus A, Schiffer CA, et al. Four years of follow-up of 1027 patients with late chronic phase (L-CP), accelerated phase (AP), or blast crisis (BC) chronic myeloid leukemia (CML) treated with imatinib in three large phase II trials. Blood 2004;104:Abstract #23.
- Visani G, Rosti G, Bandini G, Tosi P, Isidori A, Malagola M, et al. Second chronic phase before transplantation is crucial for improving survival of blastic phase chronic myeloid leukaemia. Br J Haematol 2000;109:722-8.
- Soverini S, Martinelli G, Rosti G, Bassi S, Amabile M, Poerio A, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: A study by the GIMEMA working party on chronic myeloid leukemia. J Clin Oncol 2005;23:4100-9.
- Milojkovic D, Apperley JF, Gerrard G, Ibrahim AR, Szydlo R, Bua M, et al. Responses to second-line tyrosine kinase inhibitors are durable: An intention-to-treat analysis in chronic myeloid leukemia patients. Blood 2012;119:1838-43.
- Biggs JC, Szer J, Crilley P, Atkinson K, Downs K, Dodds A, et al. Treatment of chronic myeloid leukemia with allogeneic bone marrow transplantation after preparation with BuCy2. Blood 1992;80:1352-7.
- Clift RA, Buckner CD, Thomas ED, Bryant E, Anasetti C, Bensinger WI, et al. Marrow transplantation for patients in accelerated phase of chronic myeloid leukemia. Blood 1994;84:4368-73.
- Enright H, Daniels K, Arthur DC, Dusenbery KE, Kersey JH, Kim T, et al. Related donor marrow transplant for chronic myeloid leukemia: Patient characteristics predictive of outcome. Bone Marrow Transplant 1996;17:537-42.
- Hansen JA, Gooley TA, Martin PJ, Appelbaum F, Chauncey TR, Clift RA, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med 1998;338:962-8.
- Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med 2001;344:175-81.
- Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood 2002;100:1525-31.
- Oehler VG, Radich JP, Storer B, Blume KG, Chauncey T, Clift R, et al. Randomized trial of allogeneic related bone marrow transplantation versus peripheral blood stem cell transplantation for chronic myeloid leukemia. Biol Blood Marrow Transplant 2005;11:85-92.
- Clift RA, Appelbaum FR, Thomas ED. Treatment of chronic myeloid leukemia by marrow transplantation. Blood 1993;82:1954-6.
- Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic leukemia working party of the European group for blood and marrow transplantation. Lancet 1998;352:1087-92.
- Passweg JR, Walker I, Sobocinski KA, Klein JP, Horowitz MM, Giralt SA, et al. Validation and extension of the EBMT Risk Score for patients with chronic myeloid leukaemia (CML) receiving allogeneic haematopoietic stem cell transplants. Br J Haematol 2004;125:613-20.
- Goldman JM, Szydlo R, Horowitz MM, Gale RP, Ash RC, Atkinson K, et al. Choice of pretransplant treatment and timing of transplants for chronic myelogenous leukemia in chronic phase. Blood 1993;82:2235-8.
- Shimoni A, Kröger N, Zander AR, Rowe JM, Hardan I, Avigdor A, et al. Imatinib mesylate (STI571) in preparation for allogeneic hematopoietic stem cell transplantation and donor lymphocyte infusions in patients with Philadelphia-positive acute leukemias. Leukemia 2003;17:290-7.
- Oehler VG, Gooley T, Snyder DS, Johnston L, Lin A, Cummings CC, et al. The effects of imatinib mesylate treatment before allogeneic transplantation for chronic myeloid leukemia. Blood 2007;109:1782-9.
- Kantarjian HM, O′Brien S, Cortes JE, Giralt SA, Rios MB, Shan J, et al. Imatinib mesylate therapy for relapse after allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood 2002;100:1590-5.
- Eapen M, Giralt SA, Horowitz MM, Klein JP, Wagner JE, Zhang MJ, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant 2004;34:721-7.
- Kröger N. Approaches to relapse after allogeneic stem cell transplantation. Curr Opin Oncol 2011;23:203-8.
- Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995;86:2041-50.
- Bornhäuser M, Thiede C, Platzbecker U, Kiani A, Oelschlaegel U, Babatz J, et al. Prophylactic transfer of BCR-ABL-, PR1-, and WT1-reactive donor T cells after T cell-depleted allogeneic hematopoietic cell transplantation in patients with chronic myeloid leukemia. Blood 2011;117:7174-84.
- Kolb HJ, Schmid C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood 2004;103:767-76.
- Dazzi F, Szydlo RM, Craddock C, Cross NC, Kaeda J, Chase A, et al. Comparison of single-dose and escalating-dose regimens of donor lymphocyte infusion for relapse after allografting for chronic myeloid leukemia. Blood 2000;95:67-71.
- DeAngelo DJ, Hochberg EP, Alyea EP, Longtine J, Lee S, Galinsky I, et al. Extended follow-up of patients treated with imatinib mesylate (gleevec) for chronic myelogenous leukemia relapse after allogeneic transplantation: durable cytogenetic remission and conversion to complete donor chimerism without graft-versus-host disease. Clin Cancer Res 2004;10:5065-71.
- Olavarria E, Siddique S, Griffiths MJ, Avery S, Byrne JL, Piper KP, et al. Post transplantation imatinib as a strategy to postpone the requirement for immunotherapy in patients undergoing reduced-intensity allografts for chronic myeloid leukemia. Blood 2007;110:4614-7.


 PDF
PDF  Views
Views  Share
Share

