Correlation of Hormone Receptor and Human Epidermal Growth Factor Receptor 2/neu Expression in Breast Cancer with Various Clinicopathologic Factors
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 483-489
DOI: DOI: 10.4103/ijmpo.ijmpo_98_16
Abstract
Background: A significant development in the breast carcinoma management is the correlation between the presence of hormone receptors in the tumor and response to hormonal therapy and chemotherapy. Human epidermal growth factor receptor-2/neu (Her-2/neu) overexpression also serves as a very useful parameter to predict response to herceptin. Aim of Study: The study was conducted to correlate immunohistochemical expression of markers such as estrogen receptor (ER), progesterone receptor (PR), and Her-2/neu with various clinicopathologic parameters. Materials and Methods: The study included 509 cases of breast carcinoma over a period of 5 years (from May 2009 to May 2014). Immunohistochemistry (IHC) for ER, PR, and her-2/neu was performed. Results: ER positivity was observed in 42.8% (218/509) cases, PR positivity in 31.8% (194/509) cases whereas her-2 neu positivity was seen in 40.7% (203/509) cases. Triple marker (ER, PR, and Her-2/neu) negative cases were 23.6% (120/509) cases. ER and PR expression was found to have a statistically significant correlation with tumor grade. Statistically significant correlation was observed between tumor size and tumor grade and her-2/neu expression. Her-2/neu expression showed statistically significant association with tumor stage. As the tumor grade increased, the proportion of triple-negative cases went on increasing, which was statistically significant. Conclusion: IHC has an increasingly important prognostic role in determination of factors that affect clinicopathologic features. Nevertheless, the results of this large series showed different patterns of findings with respect to clinicopathologic features.
Keywords
Breast cancer - estrogen receptor - human epidermal growth factor receptor-2/neu - hormone receptors - progesterone receptorPublication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
A significant development in the breast carcinoma management is the correlation between the presence of hormone receptors in the tumor and response to hormonal therapy and chemotherapy. Human epidermal growth factor receptor-2/neu (Her-2/neu) overexpression also serves as a very useful parameter to predict response to herceptin.
Aim of Study:
The study was conducted to correlate immunohistochemical expression of markers such as estrogen receptor (ER), progesterone receptor (PR), and Her-2/neu with various clinicopathologic parameters.
Materials and Methods:
The study included 509 cases of breast carcinoma over a period of 5 years (from May 2009 to May 2014). Immunohistochemistry (IHC) for ER, PR, and her-2/neu was performed.
Results:
ER positivity was observed in 42.8% (218/509) cases, PR positivity in 31.8% (194/509) cases whereas her-2 neu positivity was seen in 40.7% (203/509) cases. Triple marker (ER, PR, and Her-2/neu) negative cases were 23.6% (120/509) cases. ER and PR expression was found to have a statistically significant correlation with tumor grade. Statistically significant correlation was observed between tumor size and tumor grade and her-2/neu expression. Her-2/neu expression showed statistically significant association with tumor stage. As the tumor grade increased, the proportion of triple-negative cases went on increasing, which was statistically significant.
Conclusion:
IHC has an increasingly important prognostic role in determination of factors that affect clinicopathologic features. Nevertheless, the results of this large series showed different patterns of findings with respect to clinicopathologic features.
Introduction
Breast cancer is the most common malignancy in females worldwide, and more than 1 million women are diagnosed with breast cancer each year.[1] Most cases of invasive carcinoma breast are ductal in origin (over 90%). Invasive ductal carcinoma of no special type not otherwise specified (NOS) accounts for 60%–80% of all cases of breast carcinoma.[2]
In breast carcinoma, several features have prognostic significance including histologic subtype, grade, lymph node states, estrogen receptor (ER)/progesterone receptor (PR) status, human epidermal growth factor receptor-2/neu (her-2/neu) status, growth factors and its receptors, proliferative activity and DNA content, oncogenes, and tumor suppressor genes. At present, ER status is regarded as the most powerful predictive marker in the treatment of breast cancer even though ER and PR are codependent variables.[2]
In today's era, a conservative cum reconstructive surgical approach is becoming more and more popular in the treatment of breast carcinoma. A correlation between the presence of hormone receptors in the tumor and response to hormonal therapy and chemotherapy is a significant development in the breast carcinoma management. Her-2 neu overexpression also serves as a very useful parameter to predict response to herceptin, but it is not a good predictor of response to chemotherapy or overall survival.[2,3] This study was conducted to correlate immunohistochemical expression of markers such as ER, PR, and Her-2neu with various clinicopathologic parameters.
Materials and Methods
The present study included all cases of breast carcinoma (509) over a period of 5 years (from May 2009 to May 2014) retrieved from the archives of Department of Pathology of our institute. The study was approved by the Institutional Ethics Committee. Clinical characteristics of patients such as age, sex, and menopausal status were documented from case files. All the modified radical mastectomy specimens were examined grossly to look for tumor size and nodal metastasis. All tissues were fixed in 10% buffered formalin immediately after resection but not more than 24 h. Representative sections were taken from tumor and submitted for processing, and routine hematoxylin and eosin staining was performed for histopathological diagnosis. Tumors were graded according to Modified Bloom Richardson grading system.
Immunohistochemistry (IHC) for ER, PR, and her-2/neu was performed on representative blocks of paraffin-embedded tumor tissue. Four micrometers thick sections were taken on poly-L-lysine-coated slides and submitted for IHC. Antigen retrieval was done using citrate buffer at pH 2.5 for hormone receptors and pH 6 for her-2/neu. They were then incubated for 30 min with primary monoclonal antibodies against her-2 (DAKO, clone 124, 1:100), ER (DAKO, clone 1D5, 1/25), and PR (DAKO, clone PgR636, 1/50), followed by incubation with biotin-labeled secondary antibodies. The streptavidin-peroxidase complex was visualized using diaminobenzidine as a chromogenic substrate. The normal breast ducts served as internal positive control for ER/PR. Breast carcinoma with known her-2 neu overexpression served as an external positive control for her-2/neu staining.
ER or PR was considered positive when more than 1% of tumor cell nuclei were immunoreactive. ER or PR were considered negative if <1>
For interpretation of her-2/neu staining, the following method was used:[2]
- Score 0 (Negative): No staining is observed or membrane staining is observed in <10>
- Score 1+ (Negative): A faint/barely perceptible membrane staining is detected in more than 10% of the tumor cells. The cells are only stained in part of their membrane
- Score 2+ (weakly Positive): A weak-to-moderate complete membrane staining is observed in more than 10% of the tumor cells
- Score 3+ (Strongly Positive): A strong complete membrane staining is observed in more than 30% (formerly 10%) of the tumor cells
- Score 3 + was considered as positive immunostaining for her-2 neu.
Results
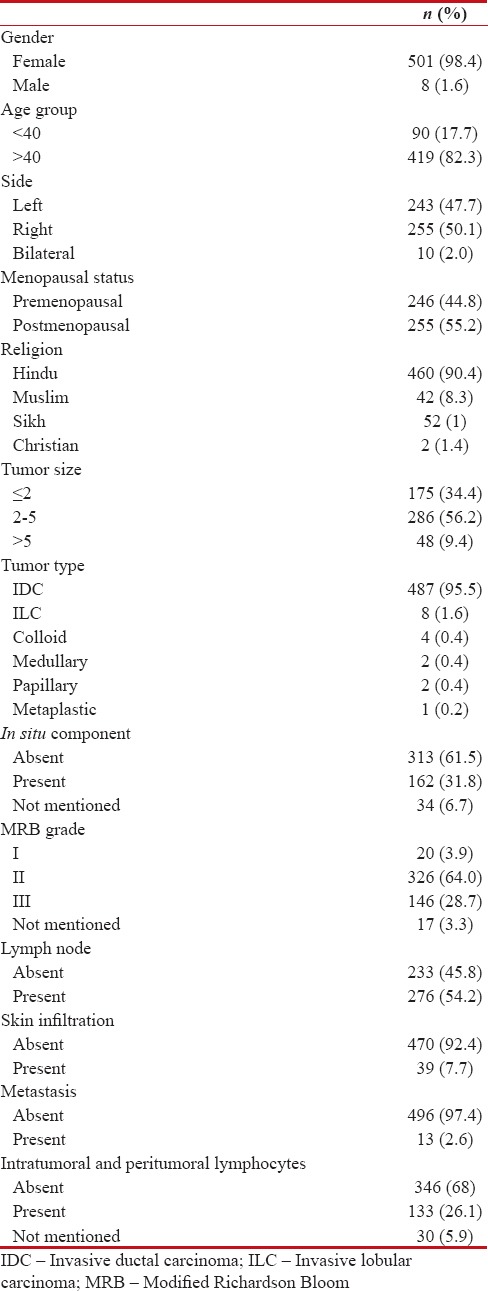
The study group comprised of a total of 509 breast carcinoma patients, majority being females (501/509; 98.4%). Out of these, 90 were below 40 years of age whereas 419 were more than 40 years of age. According to tumor size, most of the cases (56.2%) belonged to intermediate group with tumor size of 2–5 cm. The most commonly encountered histologic type was infiltrating duct carcinoma, not otherwise categorized (invasive ductal carcinoma [IDC], NOC). Most of the tumors (64%) belonged to modified Richardson–Bloom (MRB) Grade 2. Intratumoral and peritumoral lymphocytes were observed in 26.1%-cases whereas lymph node metastasis was detected in 54.2%-cases (276/509). Skin infiltration by tumor was present in 39-cases whereas distant metastases were detected in 13-cases (2.6%). The detailed clinicopathological profile of the study group is depicted in Table 1.
Table 1
Clinicopathological profile of breast carcinoma patients
|
Among the carcinoma breast patients, majority belonged to Stage II (248/509) followed by Stage III (150/509), Stage I (98/509), and Stage IV (13/509).
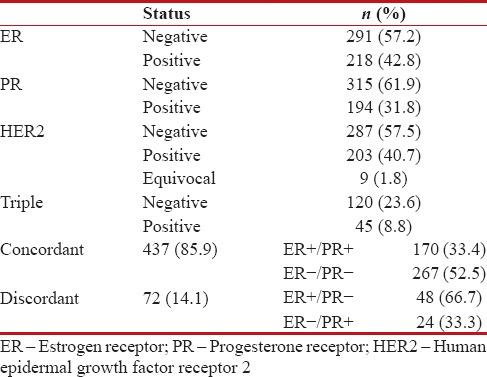
Immunohistochemical profile of the study group as shown in Table 2 revealed ER positivity in 42.8% (218/509) cases, PR positivity in 31.8% (194/509) cases whereas her-2 neu positivity seen in 40.7%-cases [Figure 1]. Triple marker (ER, PR, and Her-2/neu) negative cases were 23.6% and 8.8%-were triple marker positive. Cases in which both ER and PR showed similar results (that is, either both positive or both negative) were considered to be concordant whereas cases with one marker positive and other negative or vice versa were taken as discordant. In the present study, 85.9%-cases were concordant, of which 33.4%-showed both ER and PR positivity whereas 52.5%- showed both markers as negative. Nearly 14.1%-cases were found to show discordant results.
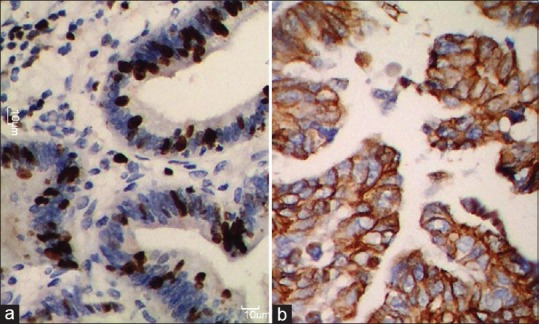
| Figure 1:(a) Photomicrograph shows strong estrogen receptor positivity in the tumor nuclei; (b) strong membranous staining for human epidermal growth factor receptor-2 (score 3+)
Table 2
Immunohistochemical profile of the study group
|
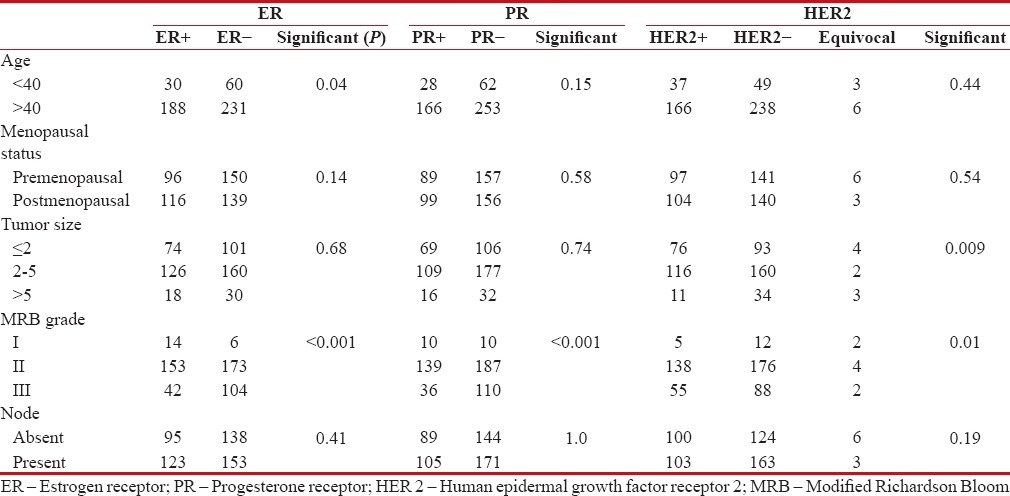
The correlation between various clinicopathological parameters and immunohistochemical profile is shown in Table 3. On correlating age with ER positivity, the difference between women <40>40 years was found to be statistically significant (P = 0.04). Moreover, as the MRB grade of the tumor increased, the ER positivity gradually decreased (P < 0>P < 0>
Table 3
Correlation between various clinicopathological parameters and immunohistochemical profile
|
Her-2/neu expression of tumor decreased with increase in the tumor size which was statistically significant (P = 0.009). Moreover, statistically significant correlation was observed between tumor grade and her-2/neu expression. There was no significant association of her-2/neu positivity with age, menopausal status, or node involvement by tumor.
On correlating the triple negative and triple positive cases with the clinicopathological parameters, it was observed that as the MRB grade of the tumor increased, the proportion of triple negative cases went on increasing (60% in Grade 1, 66.3% in Grade 2, and 86.4% in Grade 3), which was statistically significant (P = 0.01). However, none of the other parameters showed any significant association with triple marker positivity or negativity.
The correlation of various parameters with the concordant and discordant cases is shown in Table 4. The difference between ER/PR [removed]among concordant cases) between women <40>40 years was found to be statistically significant (P = 0.04). The ER/PR expression in the concordant cases also correlated significantly with MRB grade of tumor (P < 0>
Table 4
Correlation of clinicopathological parameters with the concordant and discordant cases
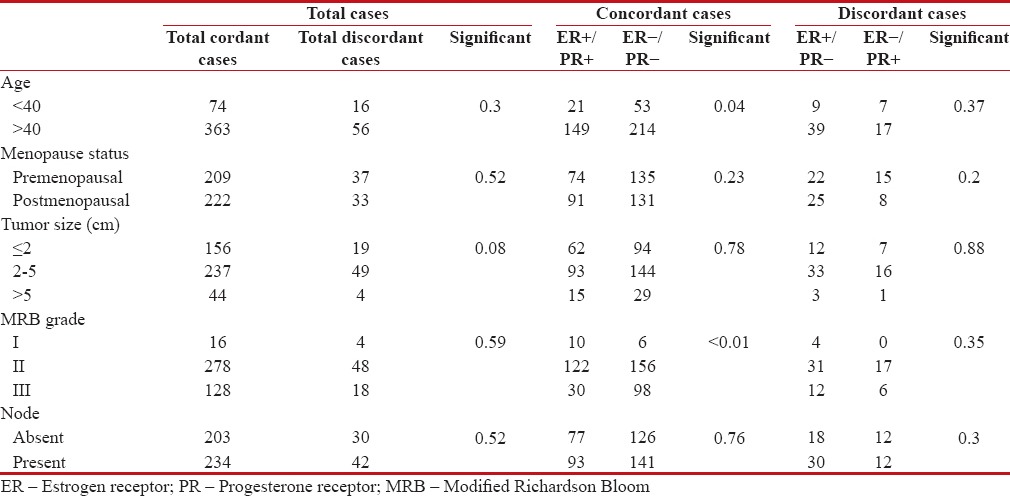
|
When the tumor stage was correlated with various immunohistochemical markers, her-2/neu expression decreased with increase in stage and the difference was statistically significant (P = 0.04). There was no significant association between ER and PR expression or triple negative cases and tumor stage.
Discussion
A correlation between the presence of hormone receptors in the tumor and response to hormonal therapy and chemotherapy is a significant development in the breast carcinoma management. At present, ER status is regarded as the most powerful predictive marker in the treatment of breast cancer even though ER and PR are codependent variables.[2,3]
Hormone receptors were initially measured by radioligand binding assay on tissue cytosol, but this has been effectively taken over by IHC. They can also be assessed by in situ hybridization and polymerase chain reaction. IHC has the advantages that it does not require fresh tissue, can be performed even on minute quantities of tissues, and is a relatively easy technique. ER is a thermolabile unstable protein, water soluble with a short half-life after surgical resection, so it is very important to ensure rapid fixation of specimen to obtain appropriate results. Several authors have attempted to standardize the technique and method of reporting to bring some semi-quantitation to the reporting of IHC. Scoring systems have been devised to express the results incorporating two features: number of tumor cell nuclei which are stained and the intensity of staining.[2,3]
Immunohistochemical profile showed ER positivity in 42.8%-cases, PR positivity in 31.8%, and Her-2neu positivity in 40.7%-cases. Immunohistochemically, 23.6% of all 509 cases were triple negative. These findings are analogous to other similar studies done by Ayadi et al.,[4] Ahmed et al.,[5] and Vasudha et al.[6] On the contrary, studies done by Lal et al.,[7] Moser Emliroise et al.,[8] Vaidhyanatha et al.,[9] and Munjal et al.[10] depicted a high percentage of Her-2/neu reactivity.
Hormone receptor expression has not been found to correlate well with histological type of breast carcinoma (ductal vs. lobular, no significant association). However, breast cancers with negative ER generally have pushing margins, Grade 3 histology, comedo type necrosis, lymphoid stroma, and central necrosis/fibrosis.[11] ER concentrations are usually lower in tumors in premenopausal women compared to postmenopausal.[12]
In the subset of patients with ER positivity, the difference between women <40>40 years was found to be statistically significant (P = 0.04). ER positivity was found to have significant relation with tumor grade. A number of studies conducted by Ayadi et al.,[4] Adebamowo et al.,[13] Lu et al.,[14] Pinto et al.,[15] Looi and Cheah,[16] and Kaptain et al.[17] support our findings. Relationship with menopausal status was not found to have a significant association with ER positivity in coherence with findings of a study conducted by Ahmed et al.[5]
No significant relation was noted between ER positivity and tumor size, similar to the studies conducted by Ahmed et al.,[5] Bamberger et al.,[18] and Kilinç and Yaldiz.[19] Similarly, no significant relation was seen between ER positivity and lymph node positivity, a finding which is supported by many studies, i.e., by Prati et al.,[20] Huang et al.,[21] Vasudha et al.,[6] and Azizun-Nisa et al.[22]
Regarding PR positivity, it had a significant association with only tumor grade while with all other clinicopathologic parameters such as age, menopausal status, tumor size, and lymph node status, no significant relation was noted.
Her-2/neu (c-erb B2) is an oncogene that encodes a transmembrane glycoprotein with tyrosine kinase activity and belongs to the epidermal growth factor receptor family. Her-2 neu overexpression has been observed in many cases of carcinoma breast; moreover, with the discovery of herceptin (trastuzumab) as a therapeutic agent, the assessment of her-2 neu amplification in all breast cancer patients has become almost mandatory. Her-2 neu overexpression can be measured by either IHC or fluorescent in situ hybridization (FISH). There is an ongoing controversy regarding the usefulness of the two techniques; however, a consensus has now been reached that the best, cost-effective approach is to begin with IHC and do grading. If the results are either 0 or 3+, there is no need to perform FISH as the results correlate with gene expression. However, 1+ or 2+ results need to be confirmed using FISH.[2,3]
Her-2 neu overexpression serves as a very useful predictor of response to herceptin, but it is not a good predictor of response to chemotherapy or overall survival.
Regarding the relationship of her-2 neu with the histological types of breast cancer, its overexpression is seen in almost all cases of high-grade ductal carcinoma in situ, in 20%–30% IDC and small percentage of invasive lobular carcinoma.[23] On the contrary, it is characteristically absent in tubular and Grade 1 carcinomas.[24]
There is an inverse correlation between her-2/neu amplification and hormone receptor (ER and PR) expression. As in our study, ER and PR reactivity inverse association with Her-2neu is supported by studies conducted by Ahmed et al.,[5] Ayadi et al.,[4] Almasii et al.,[25] Ranatunga et al.,[26] Vasudha et al.,[6] Huang et al.,[21] and Rashed et al.[27]
With regard to Her-2neu positivity, a significant relation to tumor size was noted similar to the findings of studies conducted by Almasii et al.[25] and Vasudha et al.[6] On the contrary, majority of the studies conducted by Ayadi et al.,[4] Prati et al.,[20] Aliga et al.,[28] and Huang et al.[21] do not support this finding.
Her-2neu positivity in our study showed a significant association with tumor grade; a finding supported by Rashed et al.,[27] Cho et al.,[29] and Moradi-Marjaneh et al.[30] However, Al-Moundhii et al.[31] and Yamashita et al.[32] had contradictory findings.
In our study, Her-2neu reactivity did not have a significant association with age and menopausal status, which was in accordance with Al Moundhii et al.[31] and Yamashita et al.[32] No significant correlation was observed between her-2 neu overexpression and lymph node status as documented by Vasudha et al.,[6] Huang et al.,[21] and Azizun-Nisa et al.,[22] whereas Hussein et al.[33] had contrary findings.
There is a considerable overlap between triple-negative tumors and basal-like cancers, but still these are not synonymous with each other. Only 77% of cases classified by gene expression profiling as basal like show a triple negative phenotype whereas only 72% of cases of triple negative cancers exhibit a basal-like gene expression profile. Triple-negative tumors represent a distinct category of tumors on account of usually high-grade Intraductal Carcinoma Not Otherwise Specified (IDC NOS) morphology, a high degree of aneuploidy, a greater tendency for lung and brain metastasis, and thereby exhibit a poorer prognosis. In the present study, the triple negative tumors had significant relation with tumor grade.[34]
Limitations of the present study are that IHC is not an ideal technique to assess her 2 neu, especially in the cases with weak positivity which should be confirmed by better methods such as FISH. However, in developing countries like India, IHC being universally available (at least in major centers) is still most widely used.
IHC has an increasingly important prognostic role in determination of factors that affect clinicopathologic features. This is a large series on immunohistochemical profile of carcinoma breast as well as their correlation with clinicopathological parameters in Indian population. In the present study, ER and PR expression was found to have a statistically significant correlation with tumor grade. Statistically significant correlation was observed between tumor size and tumor grade and her-2/neu expression. Her-2/neu expression showed statistically significant association with tumor stage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108.
- Rosai J, editor. Breast. In: Rosai and Ackerman's Surgical Pathology. 10th ed. New York: Elsevier; 2011. p. 1660-771.
- Ellis IO, Pinder SE, Lee AH. Tumors of the breast. In: Fletcher CD, editor. Diagnostic Histopathology of Tumors. 3rd ed. Philadelphia: Elsevier; 2007. p. 903-70.
- Ayadi L, Khabir A, Amouri H, Karray S, Dammak A, Guermazi M, et al. Correlation of HER-2 over-expression with clinico-pathological parameters in tunisian breast carcinoma. World J Surg Oncol 2008;6:112.
- Ahmed HG, Al-Adhraei MA, Al-Thobhani AK. Correlations of hormone receptors (ER and PR), Her2/neu and p53 expression in breast ductal carcinoma among Yemeni women. Open Cancer Immunol J 2011;4:1-9.
- Vasudha M, Bharti M, Prashant R. Correlation of hormone receptor & Her 2/neu expression in breast cancer: A study at tertiary care hospital in South Gujarat. Natl J Med Res 2012;2:295-8.
- Lal P, Tan LK, Chen B. Correlation of HER-2 status with estrogen and progesterone receptors and histologic features in 3,655 invasive breast carcinomas. Am J Clin Pathol 2005;123:541-6.
- Ambroise M, Ghosh M, Mallikarjuna VS, Kurian A. Immunohistochemical profile of breast cancer patients at a tertiary care hospital in South India. Asian Pac J Cancer Prev 2011;12:625-9.
- Vaidyanathan K, Kumar P, Reddy CO, Deshmane V, Somasundaram K, Mukherjee G. Erb 2 expression and its association with other biological parameter of breast cancer Indian women. Indian J Cancer 2010;47:8-15.
- Munjal K, Ambaye A, Evans MF, Mitchell J, Nandedkar S, Cooper K, et al. Immunohistochemical analysis of ER, PR, her2 and CK5/6 in infiltrative breast carcinomas in Indian patients. Asian Pac J Cancer Prev 2009;10:773-8.
- Putti TC, El-Rehim DM, Rakha EA, Paish CE, Lee AH, Pinder SE, et al. Estrogen receptor-negative breast carcinomas: A review of morphology and immunophenotypical analysis. Mod Pathol 2005;18:26-35.
- Honma N, Sakamoto G, Akiyama F, Esaki Y, Sawabe M, Arai T, et al. Breast carcinoma in women over the age of 85: Distinct histological pattern and androgen, oestrogen, and progesterone receptor status. Histopathology 2003;42:120-7.
- Adebamowo CA, Famooto A, Ogundiran TO, Aniagwu T, Nkwodimmah C, Akang EE, et al. Immunohistochemical and molecular subtypes of breast cancer in Nigeria. Breast Cancer Res Treat 2008;110:183-8.
- Lu X, Gu Y, Ding Y, Song W, Mao J, Tan J, et al. Correlation of ER, pgR, HER-2/neu, p53, and VEGF with clinical characteristics and prognosis in Chinese women with invasive breast cancer. Breast J 2008;14:308-10.
- Pinto AE, André S, Pereira T, Nóbrega S, Soares J. C-erbB-2 oncoprotein overexpression identifies a subgroup of estrogen receptor positive (ER+) breast cancer patients with poor prognosis. Ann Oncol 2001;12:525-33.
- Looi LM, Cheah PL. C-erbB-2 oncoprotein amplification in infiltrating ductal carcinoma of breast correlates to high histologic grade and loss of estrogen receptor protein. Malays J Pathol 1998;20:19-23.
- Kaptain S, Tan LK, Chen B. Her-2/neu and breast cancer. Diagn Mol Pathol 2001;10:139-52.
- Bamberger AM, Milde-Langosch K, Schulte HM, Löning T. Progesterone receptor isoforms, PR-B and PR-A, in breast cancer: Correlations with clinicopathologic tumor parameters and expression of AP-1 factors. Horm Res 2000;54:32-7.
- Kilinç N, Yaldiz M. P53, c-erbB-2 expression and steroid hormone receptors in breast carcinoma: Correlations with histopathological parameters. Eur J Gynaecol Oncol 2004;25:606-10.
- Prati R, Apple SK, He J, Gornbein JA, Chang HR. Histopathologic characteristics predicting HER-2/neu amplification in breast cancer. Breast J 2005;11:433-9.
- Huang HJ, Neven P, Drijkoningen M, Paridaens R, Wildiers H, Van Limbergen E, et al. Association between tumour characteristics and HER-2/neu by immunohistochemistry in 1362 women with primary operable breast cancer. J Clin Pathol 2005;58:611-6.
- Azizun-Nisa, Bhurgri Y, Raza F, Kayani N. Comparison of ER, PR and HER-2/neu (C-erb B 2) reactivity pattern with histologic grade, tumor size and lymph node status in breast cancer. Asian Pac J Cancer Prev 2008;9:553-6.
- Rosenthal SI, Depowski PL, Sheehan CE, Ross JS. Comparison of HER-2/neu oncogene amplification detected by fluorescence in situ hybridization in lobular and ductal breast cancer. Appl Immunohistochem Mol Morphol 2002;10:40-6.
- Oakley GJ 3rd, Tubbs RR, Crowe J, Sebek B, Budd GT, Patrick RJ, et al. HER-2 amplification in tubular carcinoma of the breast. Am J Clin Pathol 2006;126:55-8.
- Almasri NM, Al Hamad M. Immunohistochemical evaluation of human epidermal growth factor receptor 2 and estrogen and progesterone receptors in breast carcinoma in Jordan. Breast Cancer Res 2005;7:R598-604.
- ;Ratnatunga N, Liyanapathirana LV. Hormone receptor expression and her/2neu amplification in breast carcinoma in a cohort of Sri Lankans. Ceylon Med J 2007;52:133-6.
- Rashed MM, Ragab NM, Galal MK. The association of HER2/neu over expression in relation to p53 nuclear accumulation, hormonal receptor status andcommon clinicopathological prognostic parameters in a series of Egyptian women with invasive ductal carcinoma. Eur J Gen Med 2007;4:73-9.
- Ariga R, Zarif A, Korasick J, Reddy V, Siziopikou K, Gattuso P, et al. Correlation of her-2/neu gene amplification with other prognostic and predictive factors in female breast carcinoma. Breast J 2005;11:278-80.
- Cho EY, Choi YL, Han JJ, Kim KM, Oh YL. Expression and amplification of her2, EGFR and cyclin D1 in breast cancer: Immunohistochemistry and chromogenic in situ hybridization. Pathol Int 2008;58:17-25.
- Moradi-Marjaneh M, Homaei-Shandiz F, Shamsian SA, Mashhadi EZ, Hedayati-Moghadam MR. Correlation of HER2/neu over-expression, p53 protein accumulation and steroid receptor status with tumor characteristics: An Iranian study of breast cancer patients. Iran J Public Health 2008;37:19-28.
- Al-Moundhri M, Nirmala V, Al-Mawaly K, Ganguly S, Burney I, Rizvi A, et al. Significance of p53, bcl-2, and HER-2/neu protein expression in Omani Arab females with breast cancer. Pathol Oncol Res 2003;9:226-31.
- Yamashita H, Toyama T, Nishio M, Ando Y, Hamaguchi M, Zhang Z, et al. P53 protein accumulation predicts resistance to endocrine therapy and decreased post-relapse survival in metastatic breast cancer. Breast Cancer Res 2006;8:R48.
- Hussein MR, Srah A, Abdulwahed AR. Alteration of estrogen receptors, progesterone receptors and c-erB2 oncogene protein expression in ductal carcinomas of the breast. Cell Biol Int 2008;32:698-707.
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010;363:1938-48.

| Figure 1:(a) Photomicrograph shows strong estrogen receptor positivity in the tumor nuclei; (b) strong membranous staining for human epidermal growth factor receptor-2 (score 3+)
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108.
- Rosai J, editor. Breast. In: Rosai and Ackerman's Surgical Pathology. 10th ed. New York: Elsevier; 2011. p. 1660-771.
- Ellis IO, Pinder SE, Lee AH. Tumors of the breast. In: Fletcher CD, editor. Diagnostic Histopathology of Tumors. 3rd ed. Philadelphia: Elsevier; 2007. p. 903-70.
- Ayadi L, Khabir A, Amouri H, Karray S, Dammak A, Guermazi M, et al. Correlation of HER-2 over-expression with clinico-pathological parameters in tunisian breast carcinoma. World J Surg Oncol 2008;6:112.
- Ahmed HG, Al-Adhraei MA, Al-Thobhani AK. Correlations of hormone receptors (ER and PR), Her2/neu and p53 expression in breast ductal carcinoma among Yemeni women. Open Cancer Immunol J 2011;4:1-9.
- Vasudha M, Bharti M, Prashant R. Correlation of hormone receptor & Her 2/neu expression in breast cancer: A study at tertiary care hospital in South Gujarat. Natl J Med Res 2012;2:295-8.
- Lal P, Tan LK, Chen B. Correlation of HER-2 status with estrogen and progesterone receptors and histologic features in 3,655 invasive breast carcinomas. Am J Clin Pathol 2005;123:541-6.
- Ambroise M, Ghosh M, Mallikarjuna VS, Kurian A. Immunohistochemical profile of breast cancer patients at a tertiary care hospital in South India. Asian Pac J Cancer Prev 2011;12:625-9.
- Vaidyanathan K, Kumar P, Reddy CO, Deshmane V, Somasundaram K, Mukherjee G. Erb 2 expression and its association with other biological parameter of breast cancer Indian women. Indian J Cancer 2010;47:8-15.
- Munjal K, Ambaye A, Evans MF, Mitchell J, Nandedkar S, Cooper K, et al. Immunohistochemical analysis of ER, PR, her2 and CK5/6 in infiltrative breast carcinomas in Indian patients. Asian Pac J Cancer Prev 2009;10:773-8.
- Putti TC, El-Rehim DM, Rakha EA, Paish CE, Lee AH, Pinder SE, et al. Estrogen receptor-negative breast carcinomas: A review of morphology and immunophenotypical analysis. Mod Pathol 2005;18:26-35.
- Honma N, Sakamoto G, Akiyama F, Esaki Y, Sawabe M, Arai T, et al. Breast carcinoma in women over the age of 85: Distinct histological pattern and androgen, oestrogen, and progesterone receptor status. Histopathology 2003;42:120-7.
- Adebamowo CA, Famooto A, Ogundiran TO, Aniagwu T, Nkwodimmah C, Akang EE, et al. Immunohistochemical and molecular subtypes of breast cancer in Nigeria. Breast Cancer Res Treat 2008;110:183-8.
- Lu X, Gu Y, Ding Y, Song W, Mao J, Tan J, et al. Correlation of ER, pgR, HER-2/neu, p53, and VEGF with clinical characteristics and prognosis in Chinese women with invasive breast cancer. Breast J 2008;14:308-10.
- Pinto AE, André S, Pereira T, Nóbrega S, Soares J. C-erbB-2 oncoprotein overexpression identifies a subgroup of estrogen receptor positive (ER+) breast cancer patients with poor prognosis. Ann Oncol 2001;12:525-33.
- Looi LM, Cheah PL. C-erbB-2 oncoprotein amplification in infiltrating ductal carcinoma of breast correlates to high histologic grade and loss of estrogen receptor protein. Malays J Pathol 1998;20:19-23.
- Kaptain S, Tan LK, Chen B. Her-2/neu and breast cancer. Diagn Mol Pathol 2001;10:139-52.
- Bamberger AM, Milde-Langosch K, Schulte HM, Löning T. Progesterone receptor isoforms, PR-B and PR-A, in breast cancer: Correlations with clinicopathologic tumor parameters and expression of AP-1 factors. Horm Res 2000;54:32-7.
- Kilinç N, Yaldiz M. P53, c-erbB-2 expression and steroid hormone receptors in breast carcinoma: Correlations with histopathological parameters. Eur J Gynaecol Oncol 2004;25:606-10.
- Prati R, Apple SK, He J, Gornbein JA, Chang HR. Histopathologic characteristics predicting HER-2/neu amplification in breast cancer. Breast J 2005;11:433-9.
- Huang HJ, Neven P, Drijkoningen M, Paridaens R, Wildiers H, Van Limbergen E, et al. Association between tumour characteristics and HER-2/neu by immunohistochemistry in 1362 women with primary operable breast cancer. J Clin Pathol 2005;58:611-6.
- Azizun-Nisa, Bhurgri Y, Raza F, Kayani N. Comparison of ER, PR and HER-2/neu (C-erb B 2) reactivity pattern with histologic grade, tumor size and lymph node status in breast cancer. Asian Pac J Cancer Prev 2008;9:553-6.
- Rosenthal SI, Depowski PL, Sheehan CE, Ross JS. Comparison of HER-2/neu oncogene amplification detected by fluorescence in situ hybridization in lobular and ductal breast cancer. Appl Immunohistochem Mol Morphol 2002;10:40-6.
- Oakley GJ 3rd, Tubbs RR, Crowe J, Sebek B, Budd GT, Patrick RJ, et al. HER-2 amplification in tubular carcinoma of the breast. Am J Clin Pathol 2006;126:55-8.
- Almasri NM, Al Hamad M. Immunohistochemical evaluation of human epidermal growth factor receptor 2 and estrogen and progesterone receptors in breast carcinoma in Jordan. Breast Cancer Res 2005;7:R598-604.
- ;Ratnatunga N, Liyanapathirana LV. Hormone receptor expression and her/2neu amplification in breast carcinoma in a cohort of Sri Lankans. Ceylon Med J 2007;52:133-6.
- Rashed MM, Ragab NM, Galal MK. The association of HER2/neu over expression in relation to p53 nuclear accumulation, hormonal receptor status andcommon clinicopathological prognostic parameters in a series of Egyptian women with invasive ductal carcinoma. Eur J Gen Med 2007;4:73-9.
- Ariga R, Zarif A, Korasick J, Reddy V, Siziopikou K, Gattuso P, et al. Correlation of her-2/neu gene amplification with other prognostic and predictive factors in female breast carcinoma. Breast J 2005;11:278-80.
- Cho EY, Choi YL, Han JJ, Kim KM, Oh YL. Expression and amplification of her2, EGFR and cyclin D1 in breast cancer: Immunohistochemistry and chromogenic in situ hybridization. Pathol Int 2008;58:17-25.
- Moradi-Marjaneh M, Homaei-Shandiz F, Shamsian SA, Mashhadi EZ, Hedayati-Moghadam MR. Correlation of HER2/neu over-expression, p53 protein accumulation and steroid receptor status with tumor characteristics: An Iranian study of breast cancer patients. Iran J Public Health 2008;37:19-28.
- Al-Moundhri M, Nirmala V, Al-Mawaly K, Ganguly S, Burney I, Rizvi A, et al. Significance of p53, bcl-2, and HER-2/neu protein expression in Omani Arab females with breast cancer. Pathol Oncol Res 2003;9:226-31.
- Yamashita H, Toyama T, Nishio M, Ando Y, Hamaguchi M, Zhang Z, et al. P53 protein accumulation predicts resistance to endocrine therapy and decreased post-relapse survival in metastatic breast cancer. Breast Cancer Res 2006;8:R48.
- Hussein MR, Srah A, Abdulwahed AR. Alteration of estrogen receptors, progesterone receptors and c-erB2 oncogene protein expression in ductal carcinomas of the breast. Cell Biol Int 2008;32:698-707.
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010;363:1938-48.


 PDF
PDF  Views
Views  Share
Share

