Controversies on Tumor Thickness Versus Nodal Metastasis in Oral Squamous Cell Carcinomas Revealed: A Histopathologist’s Perspective
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2018; 39(01): 18-22
DOI: DOI: 10.4103/ijmpo.ijmpo_45_16
Abstract
Background:?Cervical metastasis has a tremendous impact on prognosis in patients with head and neck squamous cell carcinomas (HNSCCs). However, to date management of clinically negative neck in HNSCC is still a controversial subject. Tumor thickness (TT) is a strong predictor for lymph node involvement in oral squamous cell carcinomas (SCCs). However, controversy exists about the optimal TT cutoff point for a clinically relevant risk to the neck.?Aim and Objectives:?The aim is to evaluate the relationship between TT and the risk of cervical lymph node involvement and to determine optimal TT cutoff point for prompting prophylactic neck management.?Materials and Methods:?The clinical files and histological sections of 35 SCC (T1/T2) at buccal mucosa site from clinically determined N0 patients were retrospectively analyzed who underwent surgical treatment of their primary lesion with simultaneous neck dissection. An ocular micrometer was used to measure the TT both in exophytic and ulcerated lesions. Chi-square contingency tables were used to correlate TT and other clinicopathological parameters with metastasis in the neck.?Results:?Clinically, negative necks turned out pathologically positive in 42.8% (n?= 15/35). In the group in which tumor depth exceeded 1.5 mm, the metastatic rate was 86.7% (13/15). In contrast, when the depth of invasion was <1 class="b" xss=removed>Conclusion:?TT is a highly significant, objectively measurable prognostic factor in early stage oral cancers and elective neck therapy is indicated for tumors exceeding 1.5 mm invasion.
Keywords
Elective neck therapy - metastasis - oral squamous cell carcinoma
Publication History
23 June 2021 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:?Cervical metastasis has a tremendous impact on prognosis in patients with head and neck squamous cell carcinomas (HNSCCs). However, to date management of clinically negative neck in HNSCC is still a controversial subject. Tumor thickness (TT) is a strong predictor for lymph node involvement in oral squamous cell carcinomas (SCCs). However, controversy exists about the optimal TT cutoff point for a clinically relevant risk to the neck.?Aim and Objectives:?The aim is to evaluate the relationship between TT and the risk of cervical lymph node involvement and to determine optimal TT cutoff point for prompting prophylactic neck management.?Materials and Methods:?The clinical files and histological sections of 35 SCC (T1/T2) at buccal mucosa site from clinically determined N0 patients were retrospectively analyzed who underwent surgical treatment of their primary lesion with simultaneous neck dissection. An ocular micrometer was used to measure the TT both in exophytic and ulcerated lesions. Chi-square contingency tables were used to correlate TT and other clinicopathological parameters with metastasis in the neck.?Results:?Clinically, negative necks turned out pathologically positive in 42.8% (n?= 15/35). In the group in which tumor depth exceeded 1.5 mm, the metastatic rate was 86.7% (13/15). In contrast, when the depth of invasion was <1 class="b" xss=removed>Conclusion:?TT is a highly significant, objectively measurable prognostic factor in early stage oral cancers and elective neck therapy is indicated for tumors exceeding 1.5 mm invasion.
Keywords
Elective neck therapy - metastasis - oral squamous cell carcinoma
Introduction
Oral squamous cell carcinoma (OSCC) is the most frequent head and neck cancer.[1] In contrast to other sites of oral cancer, the incidence of the buccal carcinoma is increasing, especially in the younger age group which is related to the widespread practice of betel nut chewing placed along the buccal mucosa to induce a feeling of euphoria.[2] The metastatic dissemination of these tumors usually occurs through the lymphatic system, and level I and II neck lymph nodes are the most commonly involved.[3] The incidence of occult metastasis in neck lymph nodes in patients with clinical stages I and II squamous cell carcinoma (SCC) of the mouth ranges from 27% to 40%.[4] The presence of cervical lymph node metastasis is consistently a strong determinant of survival in patients with SCC of the oral cavity (OSCC).[5]
The high incidence of occult lymph nodal metastasis is a strong argument for the indication of elective neck dissection (END) in clinical Stages I and II oral cancers. END may be both diagnostic and therapeutic.[6] END provides pathologic information on the status of neck nodes thus helping to determine the need for additional therapy, and can also remove undetectable cancer cells lodged in the lymph vessels. However, there is a high percentage of patients who do not have metastasis in the pathological exam (pN0). Furthermore, it does have an associated morbidity and may remove or destroy a natural barrier to cancer spread.[7] The identification of factors associated with the risk of lymph node metastasis may be useful for the proper selection of patients to END.
In 1970s, Breslow established a strong link between tumor thickness (TT) and both tumor-free survival and metastasis in patients with cutaneous melanoma.[8] Mohit-Tabatabai?et al. and Spiro?et al. first applied Breslow's hypothesis regarding the link between lymph node involvement and TT to oral SCC.[9],[10] Most studies have suggested that TT, which can be considered an objective parameter of the depth of invasion within the connective tissue is a strong predictor for lymph node involvement in oral SCC.[11] Although, in regard to the critical level of tumor thickness for predicting cervical metastasis, there were variable results which ranged from 1.5 to 5 mm; controversy still exists about the optimal TT cutoff point for a clinically relevant risk to the neck of harboring microscopic disease.[12],[13]
The aim of this study was to evaluate the demographic, clinical, and pathological factors associated with the risk of occult metastasis and prognosis in patients with clinical stages I and II SCC of the buccal mucosa and to evaluate relationship between TT and the risk of cervical lymph node involvement and to determine optimal TT cutoff point for prompting prophylactic neck management.
Materials and Methods
This is a retrospective study performed by retrieving the records from pathology archives of cases reported between 2010 and 2014. Only patients diagnosed as SCC (8070/3) with cT1N0M0/cT2N0M0 at cheek mucosa (C06.0) who had primarily surgical treatment without radiotherapy or chemotherapy were recruited. Patients with carcinoma of other sites of the oral cavity were excluded from the study. All patients received removal as the primary treatment and patients had END. The tumor types were classified into superficial, exophytic, and ulcerative. Histological sections from each patient were analyzed by a single pathologist to reconfirm the initial diagnosis of SCC and to examine the type of differentiation of the tumor. Measurements for TT were made at step sections of 1?2 mm for maximum thickness. The optical micrometer was used to measure the distance (to the nearest mm) from an imaginary line reconstructing the basement membrane of the healthy mucosa to the deepest point of tumor invasion, in superficial, exophytic, and ulcerated lesions [Figure 1]. TT measured was categorized as <1> 3 mm. All levels of nodes stained with hematoxylin and eosin were re-examined microscopically for tumor invasion [Figure 2]. Chi-square test was used to correlate clinical and histopathologic parameters with lymph node metastasis. Values of P < >
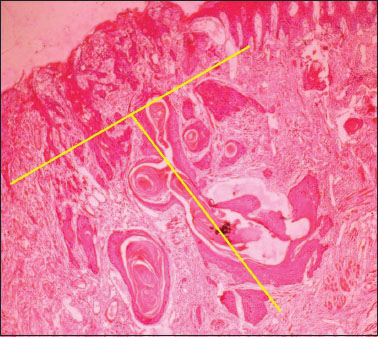
|?Figure.1Tumor thickness to the nearest 0.1 mm is determined with an ocular micrometer
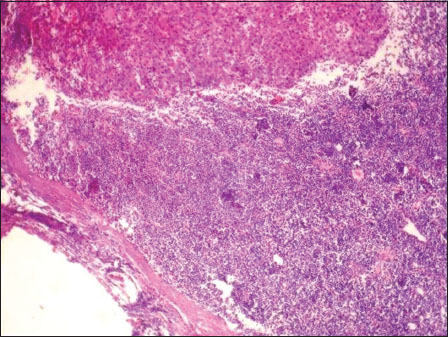
|?Figure.2Tumor invasion within the lymph node
Results
The results of the present study show a total of 35 patients were eligible for the study, 30 (85.7%) were male and 5 (14.28%) female. The patient's age ranged from 24 to 85 years (mean, 52 years). A total of 7 (20%) were clinical stage I and 28 (80%) clinical stage II. With regard to the macroscopic type, 6 (17.14%) were exophytic, 28 (80%) were ulcerative, and 1 (2.85%) was superficial type lesion. Of the 35 patients whose histologic grade was observable, 19 (54.2%) were classified as Grade I, 16 (45.7%) as Grade II, and 0 (0%) as Grade III.
Of the 35 patients who underwent END metastasis (overall occult cervical metastatic rate) in lymph nodes was found in 15/35 (42.8%). When all the clinicopathological parameters were correlated with lymph node metastases only TT correlated with nodal metastases (0.032). In the group in which TT exceeded 1.5 mm, the metastatic rate was 37% (13/35). In contrast, when the TT was equal or <1 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_45_16#TB_1" xss=removed>Table 1].{Table 1}
|
Variables |
Total number (n=35), n (%) |
Positive nodes (pN+) (n=15), n (%) |
Negative nodes (pN?) (n=20), n (%) |
P |
|---|---|---|---|---|
|
pN ? Pathological nodes |
||||
|
Sex |
||||
|
Male |
30 (85.7) |
13 (43.33) |
17 (56.67) |
0.081 |
|
Female |
5 (14.28) |
2 (40) |
3 (60) |
|
|
Age |
||||
|
<39> |
4 (11.42) |
1 (25) |
3 (75) |
0.319 |
|
40-59 |
26 (74.2) |
12 (46.15) |
14 (53.8) |
|
|
>60 |
5 (14.28) |
2 (40) |
3 (60) |
|
|
T stage |
||||
|
T1 |
7 (20) |
1 (14.28) |
6 (85.7) |
0.096 |
|
T2 |
28 (80) |
14 (50) |
14 (50) |
|
|
Tumor morphology |
||||
|
Exophytic |
6 (17.14) |
2 (33.33) |
4 (66.67) |
0.212 |
|
Ulcerative |
28 (80) |
13 (46.42) |
15 (53.57) |
|
|
Superficial |
1 (2.85) |
0 |
1 (100) |
|
|
Differentiation |
||||
|
Well |
19 (54.2) |
5 (26.31) |
14 (73.68) |
0.276 |
|
Moderate |
16 (45.7) |
10 (62.5) |
6 (37.5) |
|
|
Poorly |
0 |
0 |
0 |
|
|
Depth of invasion |
||||
|
<1> |
22 (62.8) |
2 (9.09) |
20 (90.9) |
0.032 |
|
1.6-3.5 |
13 (37.1) |
13 (100) |
0 |
|
|
>3.6 |
0 |
0 |
0 |
|
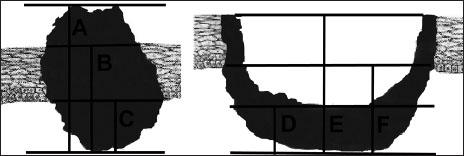
|?Figure 3Methods of measuring Tumor thickness (A-D) tumor surface/base of the ulcer-deepest point of invasion (B-E) adjacent intact mucosa-deepest point of invasion (C-F) basal membrane-deepest point of invasion
Earlier studies have shown different values due to imprecise definition of TT used between studies and as summarized by Pentenero?et al.[12],[13],[20] As mentioned in the present study measurements for TT were made using an optical micrometer by measuring the distance from an imaginary line reconstructing the basement membrane of the healthy mucosa to the deepest point of tumor invasion.
The value of 1.5 mm was taken as cutoff because 37% of cases showed metastases beyond this thickness. This may be attributed to the methodology (measurement technique) we have considered. The thickness cutoff can be more if the type of measurement technique is different like from the adjacent intact mucosa and the deepest point of invasion/from surface/base of the ulcer to the deepest point of invasion.
Alternately, some studies related the critical thickness to the site, but to date, there is no agreement about this.[20] In the present study, all the patients were with tumor in the gingival buccal site. Woolgar and Scott reported different cutoff values for TT as related to the tumor site. A possible explanation might be related to the difference in the depth and caliber of the lymphatics at the two sites.[22] O'Brien?et al. found no difference among 145 cancers from different oral cavity sites, with a median TT that was similar for the tongue, the floor of the mouth and other sites.[23]
The drawback of using the TT parameter include the absence of mucosa in some samples, the tangential cutting of some tissue sections and samples that are inadequate to allow measurement of the maximum tumor depth.[24] Surgical excision of the primary tumor and measurement of the depth of invasion by frozen section may provide additional useful information for determining the need for neck dissection in the clinically N0 patient.[15]
Conclusion
As evident from above study the optimal cutoff point for TT is 1.5 mm for oral cavity tumors in buccal mucosa site, and for tumors thicker than 1.5 mm prophylactic neck management is recommended. Although there is substantial agreement among authors despite the lack of comparable study groups, of measurement techniques, and cutoff values paradoxically enforced its reliability. Further studies are clearly awaited to reach a consensus on the topic to develop therapy protocols that are also based on this parameter by incorporating into clinical TNM staging system.
Conflict of Interest
There are no conflicts of interest.
References
- imenta AmaralTM, Da Silva FreireAR, Carvalho AL, Pinto CA, Kowalski LP.?Predictive factors of occult metastasis and prognosis of clinical stages I and II squamous cell carcinoma of the tongue and floor of the mouth. Oral Oncol 2004; 40: 780-6
- ssig H, Warraich R, Zulfiqar G, Rana M, Eckardt AM, Gellrich NC.?et al.?Assessment of cervical lymph node metastasis for therapeutic decision-making in squamous cell carcinoma of buccal mucosa: A prospective clinical analysis. World J Surg Oncol 2012; 10: 253
- eemans CR, Tiwari R, Nauta JJ, van der WaalI, Snow GB.?Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer 1994; 73: 187-90
- eski-S?ntti H, Atula T, T?rnwall J, Koivunen P, M?kitie A.?Elective neck treatment versus observation in patients with T1/T2 N0 squamous cell carcinoma of oral tongue. Oral Oncol 2006; 42: 96-101
- hingaki S, Takada M, Sasai K, Bibi R, Kobayashi T, Nomura T.?et al.?Impact of lymph node metastasis on the pattern of failure and survival in oral carcinomas. Am J Surg 2003; 185: 278-84
- uang SH, Hwang D, Lockwood G, Goldstein DP, O'Sullivan B.?Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: A meta-analysis of reported studies. Cancer 2009; 115: 1489-97
- nercl M, Yilmaz T, Gediko?lu G.?Tumor thickness as a predictor of cervical lymph node metastasis in squamous cell carcinoma of the lower lip. Otolaryngol Head Neck Surg 2000; 122: 139-42
- reslow A.?et al.?Tumor thickness, level of invasion and node dissection in stage I cutaneous melanoma. Ann Surg 1975; 182: 572-5
- ?Mohit-Tabatabai MA, Sobel HJ, Rush BF, Mashberg A.?Relation of thickness of floor of mouth stage I and II cancers to regional metastasis. Am J Surg 1986; 152: 351-3
- Spiro RH, Huvos AG, Wong GY, Spiro JD, Gnecco CA, Strong EW.?et al.?Predictive value of tumor thickness in squamous carcinoma confined to the tongue and floor of the mouth. Am J Surg 1986; 152: 345-50
- Clark JR, Naranjo N, Franklin JH, de AlmeidaJ, Gullane PJ.?Established prognostic variables in N0 oral carcinoma. Otolaryngol Head Neck Surg 2006; 135: 748-53
- Po Wing YuenA, Lam KY, Lam LK, Ho CM, Wong A, Chow TL.?et al.?Prognostic factors of clinically stage I and II oral tongue carcinoma-A comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez-Gimeno score, and pathologic features. Head Neck 2002; 24: 513-20
- Candela FC, Kothari K, Shah JP.?Patterns of cervical node metastases from squamous carcinoma of the oropharynx and hypopharynx. Head Neck 1990; 12: 197-203
- DiTroia JF.?Nodal metastases and prognosis in carcinoma of the oral cavity. Otolaryngol Clin North Am 1972; 5: 333-42
- Fukano H, Matsuura H, Hasegawa Y, Nakamura S.?Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck 1997; 19: 205-10
- Rasgon VM, Cruz RM, Hilsinger RLJr, Sawicki JE.?Relation of lymph-node metastasis to histopathologic appearance in oral cavity and oropharyngeal carcinoma: A case series and literature review. Laryngoscope 1989; 99: 1103-10
- Mendelson BC, Woods JE, Beahrs OH.?Neck dissection in the treatment of carcinoma of the anterior two-thirds of the tongue. Surg Gynecol Obstet 1976; 143: 75-80
- Byers RM, Wolf PF, Ballantyne AJ.?Rationale for elective modified neck dissection. Head Neck Surg 1988; 10: 160-7
- Shintani S, Matsuura H, Hasegawa Y, Nakayama B, Fujimoto Y.?The relationship of shape of tumor invasion to depth of invasion and cervical lymph node metastasis in squamous cell carcinoma of the tongue. Oncology 1997; 54: 463-7
- Pentenero M, Gandolfo S, Carrozzo M.?Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: A review of the literature. Head Neck 2005; 27: 1080-91
- Moore C, Kuhns JG, Greenberg RA.?Thickness as prognostic aid in upper aerodigestive tract cancer. Arch Surg 1986; 121: 1410-4
- Woolgar JA, Scott J.?Prediction of cervical lymph node metastasis in squamous cell carcinoma of the tongue/floor of mouth. Head Neck 1995; 17: 463-72
- O'Brien CJ, Lauer CS, Fredricks S, Clifford AR, McNeil EB, Bagia JS.?et al.?Tumor thickness influences prognosis of T1 and T2 oral cavity cancer ? But what thickness?. Head Neck 2003; 25: 937-45
- Gonzalez-Moles MA, Esteban F, Rodriguez-Archilla A, Ruiz-Avila I, Gonzalez-Moles S.?Importance of tumour thickness measurement in prognosis of tongue cancer. Oral Oncol 2002; 38: 394-7
Address for correspondence
Publication History
23 June 2021 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Figure.1Tumor thickness to the nearest 0.1 mm is determined with an ocular micrometer

|?Figure.2Tumor invasion within the lymph node

|?Figure 3Methods of measuring Tumor thickness (A-D) tumor surface/base of the ulcer-deepest point of invasion (B-E) adjacent intact mucosa-deepest point of invasion (C-F) basal membrane-deepest point of invasion
References
- Da Silva FreireAR, Carvalho AL, Pinto CA, Kowalski LP.?Predictive factors of occult metastasis and prognosis of clinical stages I and II squamous cell carcinoma of the tongue and floor of the mouth. Oral Oncol 2004; 40: 780-6
- H, Warraich R, Zulfiqar G, Rana M, Eckardt AM, Gellrich NC.?et al.?Assessment of cervical lymph node metastasis for therapeutic decision-making in squamous cell carcinoma of buccal mucosa: A prospective clinical analysis. World J Surg Oncol 2012; 10: 253
- Tiwari R, Nauta JJ, van der WaalI, Snow GB.?Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer 1994; 73: 187-90
- H, Atula T, T?rnwall J, Koivunen P, M?kitie A.?Elective neck treatment versus observation in patients with T1/T2 N0 squamous cell carcinoma of oral tongue. Oral Oncol 2006; 42: 96-101
- Takada M, Sasai K, Bibi R, Kobayashi T, Nomura T.?et al.?Impact of lymph node metastasis on the pattern of failure and survival in oral carcinomas. Am J Surg 2003; 185: 278-84
- SH, Hwang D, Lockwood G, Goldstein DP, O'Sullivan B.?Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: A meta-analysis of reported studies. Cancer 2009; 115: 1489-97
- Yilmaz T, Gediko?lu G.?Tumor thickness as a predictor of cervical lymph node metastasis in squamous cell carcinoma of the lower lip. Otolaryngol Head Neck Surg 2000; 122: 139-42
- A.?et al.?Tumor thickness, level of invasion and node dissection in stage I cutaneous melanoma. Ann Surg 1975; 182: 572-5
- ohit-Tabatabai MA, Sobel HJ, Rush BF, Mashberg A.?Relation of thickness of floor of mouth stage I and II cancers to regional metastasis. Am J Surg 1986; 152: 351-3
- Huvos AG, Wong GY, Spiro JD, Gnecco CA, Strong EW.?et al.?Predictive value of tumor thickness in squamous carcinoma confined to the tongue and floor of the mouth. Am J Surg 1986; 152: 345-50
- Naranjo N, Franklin JH, de AlmeidaJ, Gullane PJ.?Established prognostic variables in N0 oral carcinoma. Otolaryngol Head Neck Surg 2006; 135: 748-53
- ing YuenA, Lam KY, Lam LK, Ho CM, Wong A, Chow TL.?et al.?Prognostic factors of clinically stage I and II oral tongue carcinoma-A comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez-Gimeno score, and pathologic features. Head Neck 2002; 24: 513-20
- FC, Kothari K, Shah JP.?Patterns of cervical node metastases from squamous carcinoma of the oropharynx and hypopharynx. Head Neck 1990; 12: 197-203
- JF.?Nodal metastases and prognosis in carcinoma of the oral cavity. Otolaryngol Clin North Am 1972; 5: 333-42
- Matsuura H, Hasegawa Y, Nakamura S.?Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck 1997; 19: 205-10
- Cruz RM, Hilsinger RLJr, Sawicki JE.?Relation of lymph-node metastasis to histopathologic appearance in oral cavity and oropharyngeal carcinoma: A case series and literature review. Laryngoscope 1989; 99: 1103-10
- BC, Woods JE, Beahrs OH.?Neck dissection in the treatment of carcinoma of the anterior two-thirds of the tongue. Surg Gynecol Obstet 1976; 143: 75-80
- Wolf PF, Ballantyne AJ.?Rationale for elective modified neck dissection. Head Neck Surg 1988; 10: 160-7
- S, Matsuura H, Hasegawa Y, Nakayama B, Fujimoto Y.?The relationship of shape of tumor invasion to depth of invasion and cervical lymph node metastasis in squamous cell carcinoma of the tongue. Oncology 1997; 54: 463-7
- M, Gandolfo S, Carrozzo M.?Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: A review of the literature. Head Neck 2005; 27: 1080-91
- Kuhns JG, Greenberg RA.?Thickness as prognostic aid in upper aerodigestive tract cancer. Arch Surg 1986; 121: 1410-4
- JA, Scott J.?Prediction of cervical lymph node metastasis in squamous cell carcinoma of the tongue/floor of mouth. Head Neck 1995; 17: 463-72
- CJ, Lauer CS, Fredricks S, Clifford AR, McNeil EB, Bagia JS.?et al.?Tumor thickness influences prognosis of T1 and T2 oral cavity cancer ? But what thickness?. Head Neck 2003; 25: 937-45
- Gonzalez-Moles MA, Esteban F, Rodriguez-Archilla A, Ruiz-Avila I, Gonzalez-Moles S.?Importance of tumour thickness measurement in prognosis of tongue cancer. Oral Oncol 2002; 38: 394-7


 PDF
PDF  Views
Views  Share
Share

