Comparison of the efficacy and safety of Rituximab (Mabthera™) and its biosimilar (Reditux™) in diffuse large B-cell lymphoma patients treated with chemo-immunotherapy: A retrospective analysis
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(04): 292-298
DOI: DOI: 10.4103/0971-5851.125248
Abstract
Background: Rituximab (Mabthera™) have been in use in India since 2000. A biosimilar molecule of rituximab (Reditux™) was approved in India in 2007. This retrospective audit was done to compare the efficacy and safety of Mabthera™ with Reditux™. Materials and Methods: We reviewed the charts of 223 adult diffuse large B-cell lymphoma patients who had received cyclophosphamide, doxorubicin, vincristine and prednisolone with rituximab chemotherapy. Tumor recurrence, survival and toxicities experienced during chemotherapy were obtained from the patient charts. The survival analysis was restricted to patients who received at least 4 cycles of the same brand. Results: Of the 223 patients evaluated, 101 received Mabthera™, 72 received Reditux™. There were no differences in the infusional reaction rates, grades 3 and 4 neutropenia and oral mucositis between the two brands. Complete-remission (CR) rates were similar with Mabthera™ and Reditux™ (75% and 82%, respectively; P = 0.294). The progression free survival (PFS) rate at 5 years were 72% in Mabthera™ and 81% in Reditux™ (P = 0.382). The overall survival (OS) at 5 years were comparable in the two groups (66% in Mabthera™ and 76% in Reditux™; P = 0.264). Conclusion: We observed no significant differences in the toxicity, tumor response rates, PFS and OS between the two available brands of rituximab.
Keywords
Anti-CD20 - biosimilar - lymphoid neoplasms - monoclonal antibody - observational study - survival outcomesPublication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Rituximab (Mabthera™) have been in use in India since 2000. A biosimilar molecule of rituximab (Reditux™) was approved in India in 2007. This retrospective audit was done to compare the efficacy and safety of Mabthera™ with Reditux™.
Materials and Methods:
We reviewed the charts of 223 adult diffuse large B-cell lymphoma patients who had received cyclophosphamide, doxorubicin, vincristine and prednisolone with rituximab chemotherapy. Tumor recurrence, survival and toxicities experienced during chemotherapy were obtained from the patient charts. The survival analysis was restricted to patients who received at least 4 cycles of the same brand.
Results:
Of the 223 patients evaluated, 101 received Mabthera™, 72 received Reditux™. There were no differences in the infusional reaction rates, grades 3 and 4 neutropenia and oral mucositis between the two brands. Complete-remission (CR) rates were similar with Mabthera™ and Reditux™ (75% and 82%, respectively; P = 0.294). The progression free survival (PFS) rate at 5 years were 72% in Mabthera™ and 81% in Reditux™ (P = 0.382). The overall survival (OS) at 5 years were comparable in the two groups (66% in Mabthera™ and 76% in Reditux™; P = 0.264).
Conclusion:
We observed no significant differences in the toxicity, tumor response rates, PFS and OS between the two available brands of rituximab.
INTRODUCTION
Rituximab along with multi-agent chemotherapy [cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP)] has been the standard of care for diffuse large B-cell lymphoma (DLBCL) patients including elderly[1,2] and young patients.[3] Trials (GELA LNH 98-5[1] and MInT trials[3]) have proven the benefit of adding rituximab to CHOP in terms of improved overall response rates (ORR), prolonged progression free survival (PFS) and overall survival (OS). Rituximab was launched as Mabthera™ in USA for the treatment of B-cell non-Hodgkin's lymphoma in 1997. Food and Drug Administration approval for Mabthera™ was received in June 1998. Mabthera™ was marketed in India in early 2000 and is being used as the standard of care for DLBCL as well as low grade lymphomas.[4] However, cost of Mabthera™ is a limiting factor for its use.
A similar biologic medicinal product, commonly referred to as biosimilar, is a copy version of an approved original biologic medicine.[5] There have been frequent concerns raised about biosimilars in the medical fraternity. Since the implementation of a biosimilar approval pathway in 2005, several biosimilars have been developed.[6]
A biosimilar molecule (Reditux™) was developed by Dr. Reddy's Laboratories, Hyderabad, India and was licensed for clinical use in India, in 2007.[7,8] Thereafter, most of the oncologists in India are using the biosimilar, Reditux™ for the treatment of DLBCL in patients who were unable to afford the Mabthera™.
Biosimilars, being complex proteins, have several other aspects that need to be addressed. The international regulations regarding the testing and approval of biosimilars have been evolving and vary widely throughout the world. Comparisons of clinical outcomes [complete remission (CR), progression free survival (PFS) and overall survival (OS)] have not been done between patients receiving Mabthera™ or Reditux™. The need for post marketing surveillance and potentially large, post marketing studies has been highlighted in the literature.[6] This retrospective study was carried out to compare the efficacy, safety and toxicity of Mabthera™ with a biosimilar (Reditux™) in patients with DLBCL receiving the standard three weekly CHOP with rituximab (CHOP-R) therapies.
MATERIALS AND METHODS
This study was approved by Hospital Ethics Committee. A waiver for informed consent was obtained since the patients had already received treatment prior to initiating this study. This is a single center, retrospective, observational study. The list of all patients who received rituximab for any indication from the pharmacy records between 2004 and 2010 was retrieved. Then list of all patients diagnosed to have DLBCL from the lymphoma clinic registry during the same period was prepared.
Duration of the study
All DLBCL patients who had received CHOP-R between January 2004 and June 2010.
All adult patients with DLBCL of any stage who had received either of the two brands of rituximab along with CHOP chemotherapy were included. Both brands of rituximab (R) were administered at a standard dose of 375 mg/m2 along with CHOP regimen. CHOP regimen consisted of cyclophosphamide (C) 750 mg/m2, doxorubicin (H) 50 mg/m2, vincristine (O) 1.4 mg/m2 on day 1 and prednisone (P) 100 mg given orally on days 1-5, given every 3 weeks.
The demographic, clinical features, baseline hematological and biochemical parameters and staging were recorded. Any toxicity (hematological and non-hematological) developing after each cycle of chemotherapy documented in the case charts was extracted. Dose modifications if any were recorded for all patients. Response assessment and follow-up data was captured from case files and hospital electronic data bases.
Statistics
All analysis involved comparing the outcomes of patients receiving CHOP with either Reditux™ or Mabthera™. The important outcomes included: Response rates, grade 3 and 4 hematological and non-hematological toxicities, PFS and OS. Grade 3 and 4 hematological and all non-hematological toxicities were recorded according to the criteria of common toxicity criteria version 3.0 - Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0); Publish Date: August 9, 2006; National Institutes of Health, National Cancer Institute.
Response was defined as complete-remission (CR), partial remission (PR), stable disease and progressive disease (PD) according to the proposed International Workshop Criteria (Cheson's criteria).[9] OS was calculated from the date of diagnosis to date of death due to any cause or last follow-up. PFS was calculated from the date of diagnosis to date of tumour progression or relapse, death or last follow-up.
The Kaplan-Meier method was used to estimate response rates, PFS and OS, with differences compared by the two-sided log rank test.
Initial analysis compared all patients who received all cycles of Mabthera™ with those who received all cycles of Reditux™. A total of 50 patients had received either both the brands (29) or brand was not known (21). As 29 patients had received both the brands, we re-analyzed the data by grouping those receiving ≥4 cycles of Mabthera™ in Mabthera™ group and those receiving >4 cycles Reditux™ in Reditux™ group. We also made a comparison of the outcomes limited to patients treated from 2007 to June 2010 as Reditux™ got approval only in 2007.
RESULTS
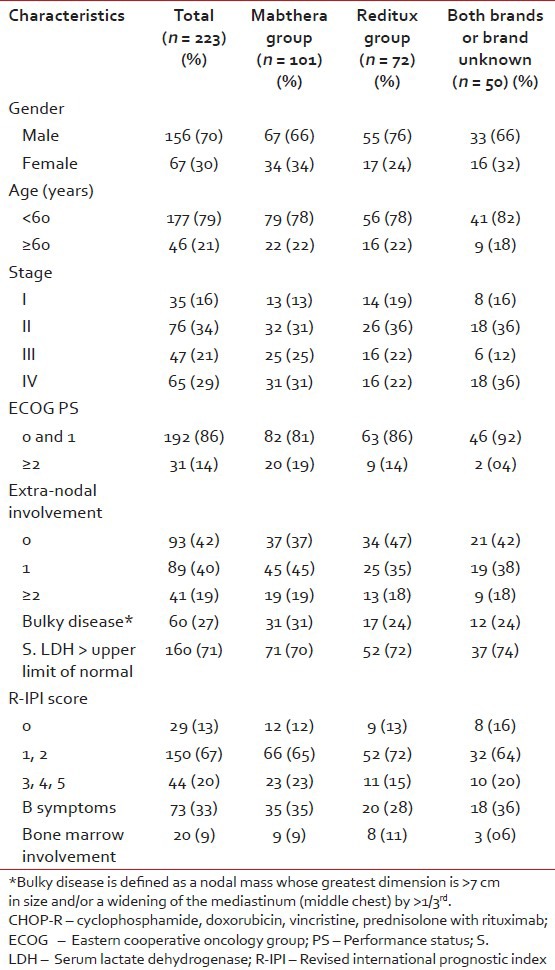
Among the 464 DLBCL patients registered in the lymphoma clinic between 2004 and 2010, 223 patients received chemo-immunotherapy with CHOP-R. The remaining 241 were excluded for various reasons: 163 received CHOP alone, 21 received etoposide based regimen, 2 patients received hyper-cyclophosphamide, vincristine, adriamycin, and dexamethasone, 8 for palliative treatment alone, 45 patients did not follow-up after treatment planning, and 2 died of tumor lysis. The demographic clinical and pathological characteristics of patients treated with CHOP-R are summarized in [Table 1].
Table 1
Baseline characteristics of all patients treated with CHOP-R
Response rate
The response rates for the whole group treated with CHOP-R were: CR rate observed was 75% (168) and PR rate was 15% (34). Nine patients (4%) had PD during treatment. In 12 patients, the response was not evaluated: Six patients died and 6 patients were lost to follow-up during therapy, before the planned response evaluation after 4 cycles.
Median number of treatment cycles delivered were 6 (range 4-8). 94% (95/101) patients in Mabthera™ group and 96% (69/72) patients in Reditux™ group received the planned chemotherapy cycles. Best response could not be evaluated in 6 patients on the Mabthera™ group (3 died during chemotherapy and 3 were lost to follow-up early during treatment). Similarly, response was not evaluated in 4 patients on the Reditux™ group (3 died on treatment and one patient was lost to follow-up).
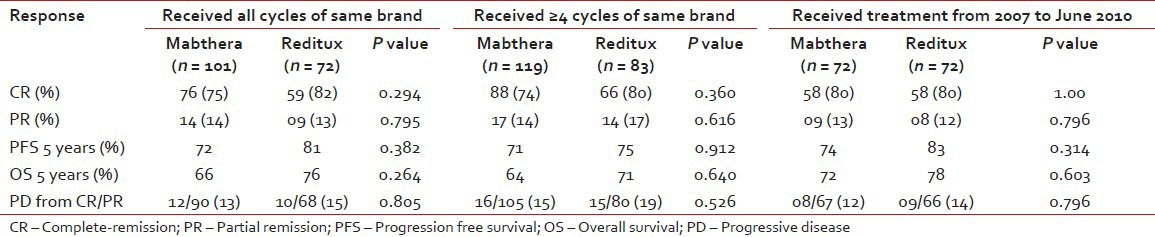
The ORR in the Mabthera™ group (CR in 76/101 patients and PR in 14/101 patients) was 89%, while it was 95% (CR in 59/72 patients and PR in 09/72 patients) in the ORR in the Reditux™ group, as shown in Table 2. Nine patients had progressive disease while on chemotherapy (five in Mabthera™ group and 4 in the combined group). Of those who were attained response to therapy (CR or PR), 12 patients (13%) in Mabthera™ group and 10 patients (15%) in Reditux™ group had progressive disease post therapy [Table 2].
Table 2
Response to therapy in all three groups of patient analysed
The number of patients, who received Mabthera™ alone or Reditux™ alone in all cycles of CHOP from 2004 to June 2010, is shown in [Figure 1].
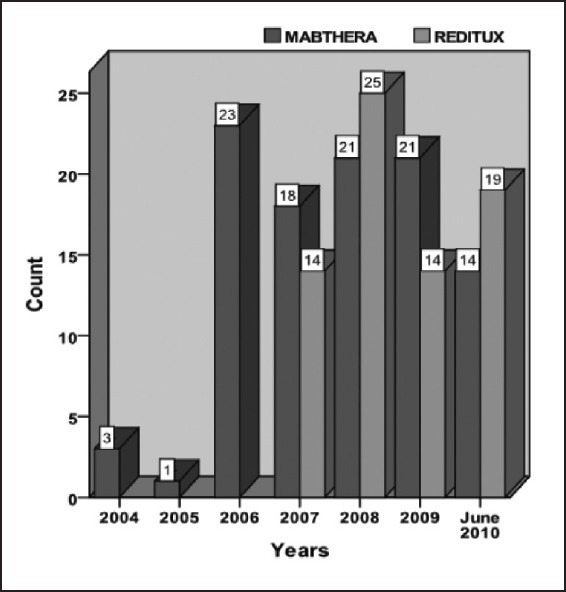
| Fig. 1 The number of patients treated with Mabthera™ or Reditux™ year-wise from 2004 to June 2010
Response analysis of those who received ≥4 cycles of either Mabthera™ or Reditux™ (response rates 88% and 97% for Mabthera™ and Reditux™, respectively) and analysis for patients who received treatment from 2007 to 2010 (83% and 92% for Mabthera™ and Reditux™, respectively) showed similar response rates.
The median follow-up of all patients was 28 months (range 0-87 months). Median period of follow-up of patients in Mabthera™ group was 33 months (range 0-87 months) and in Reditux™ group was 25 months (range 2-58 months), respectively.
Survival
At 5-year PFS for all patients treated with CHOP-R was 68% [Figure 2a]. The 5-year PFS in Mabthera™ group and Reditux™ groups were 72% and 81% respectively (P = 0.392) [Figure 2b]. The 5-years OS for all the patients was 65% [Figure 3a]; and that for Mabthera™ and Reditux™ groups were 66% and 76% respectively (P = 0.264) [Figure 3b].
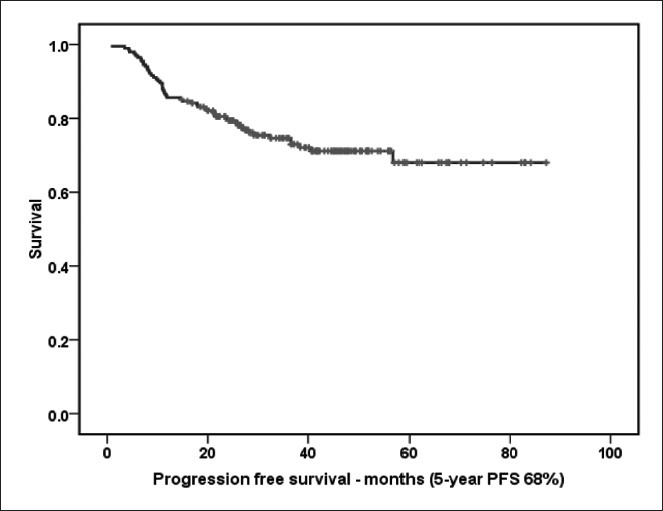
| Fig. 2a The 5-year progression free survival (months) for all patients trated with cyclophosphamide, doxorubicin, vincristine and prednisolone with rituximab
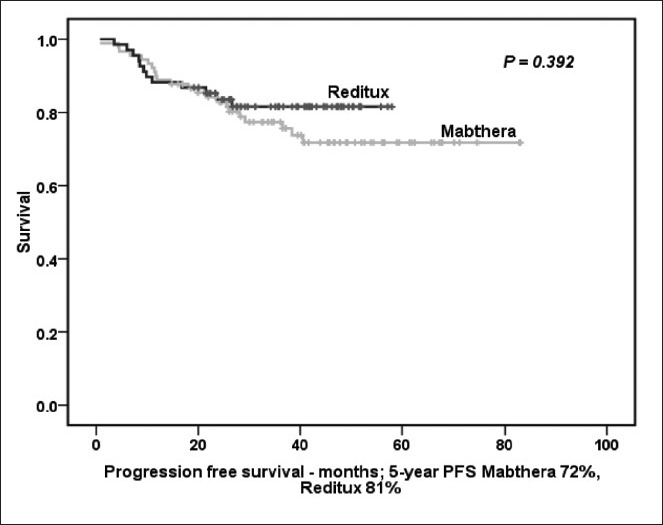
| Fig. 2b 5-year progression free survival (months) for patients receiving Mabthera™ alone or Reditux™ alone in all cycles of cyclophosphamide, doxorubicin, vincristine and prednisolone with rituximab
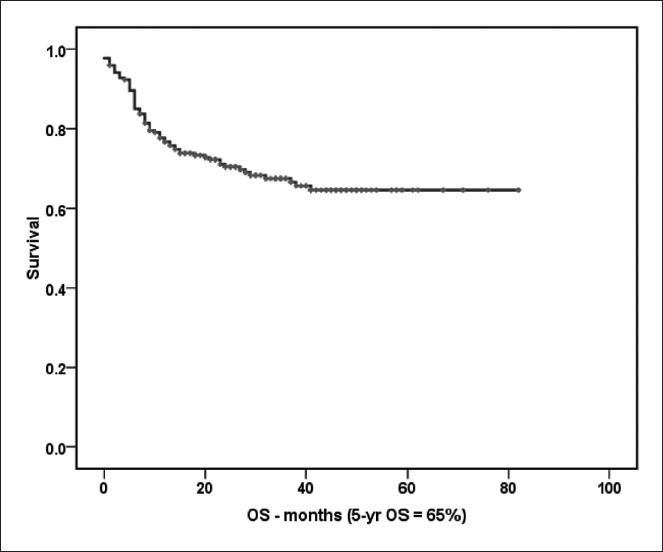
| Fig. 3a The 5-year overall survival (months) of all patients treated with cyclophosphamide, doxorubicin, vincristine and prednisolone with rituximab
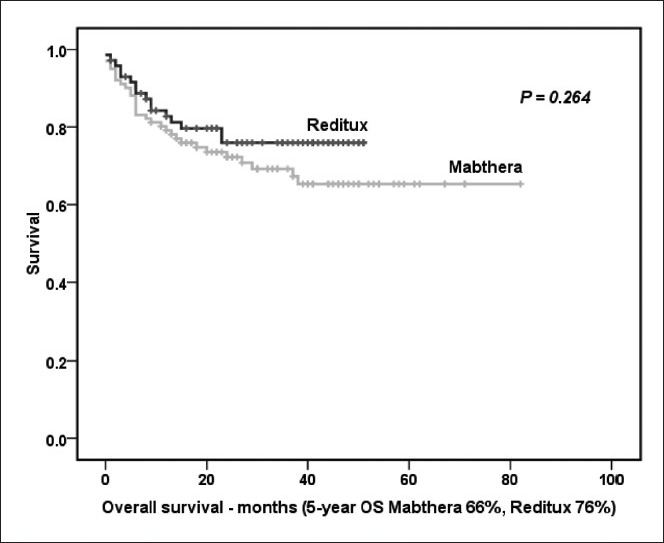
| Fig. 3b The 5-year overall survival (months) of all patients treated with Mabthera™ alone or Reditux™ alone in all cycles of cyclophosphamide, doxorubicin, vincristine and prednisolone with rituximab
Among patients who received both the brands, those who received ≥4 cycles of Mabthera™ (18) were analyzed with Mabthera™ group (119) and accordingly those who received ≥4 cycles of Reditux™ (11) were analyzed with the Reditux™ group (83). It was observed that, even in the respective combined groups (patients who received both the brands), the survival rates were similar to that seen in Mabthera™ alone and Reditux™ alone groups. The 5-year PFS in Mabthera™ combined group and Reditux™ combined group was 71% and 75%, respectively (P = 0.912). Observed 5 years OS for Mabthera™ combined group was 64% and 71% for Reditux™ combined group; (P = 0.640).
Patients of DLBCL who received CHOP-R from March 2007 to June 2010 were also analyzed. Seventy two patients received Mabthera™ and another 72 patients received Reditux™ during this time period. The 5-years PFS for all patients was 75% (73% in Mabthera™ group and 83% in Reditux™ group; P = 0.314) [Figure 4a]. For all patients 5 years OS was 71% (72% in Mabthera™ group and 78% in Reditux™ group; P = 0.603) [Figure 4b].
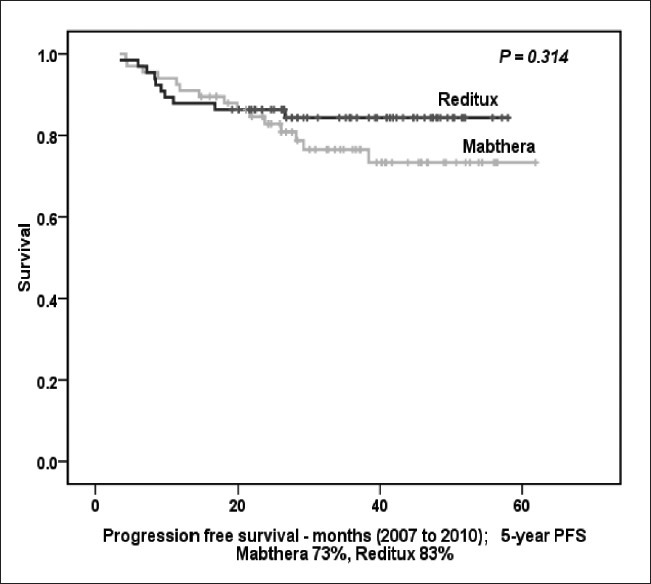
| Fig. 4a The 5-year progression free survival (months) for all patients treated from 2007 to June 2010
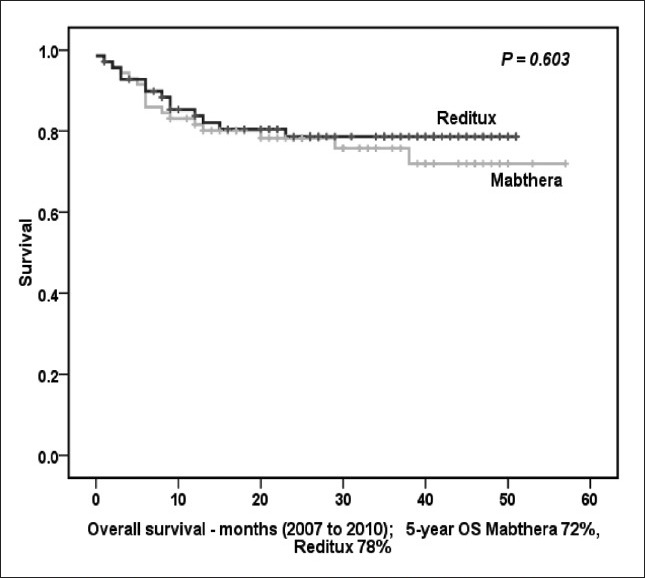
| Fig. 4b The 5-year overall survival (months) for all patients treated from 2007 to June 2010
Toxicity
Of 223 evaluable patients, toxicity data was available in a limited number of patients (Mabthera™ 40 and Reditux™ 30) from the case records. The data is presented in Table 3. Both hematological and non-hematological toxic effects were similar in the two groups. Grade 3 and 4 febrile neutropenia was observed in 9 patients in the Mabthera™ group and in 6 patients in the Reditux™ group (23% vs. 20%; P = 0.801). Three patients had developed a chest infection (pneumonia). All the patients except 2 patients in Mabthera™ group and one patient in Reditux™ group had completed the planned course of chemotherapy. Grade 3 and 4 oral mucositis was observed in 2 patients in Mabthera™ group and in 3 patients in Reditux™ group (5% vs. 10%; P = 0.385). Grade 3 and 4 diarrhea was seen in 8 and 3 patients in Mabthera™ and Reditux™ groups respectively. Incidence of peripheral neuropathy was comparable in both groups. No differences were observed in severe infusional reactions in the two groups (two patients in each groups, 5% vs. 7%; P = 0.583). All the patients responded to symptomatic therapy without any deaths in either of the groups.
Table 3
Toxicity evaluation (evaluated in 93 cases)
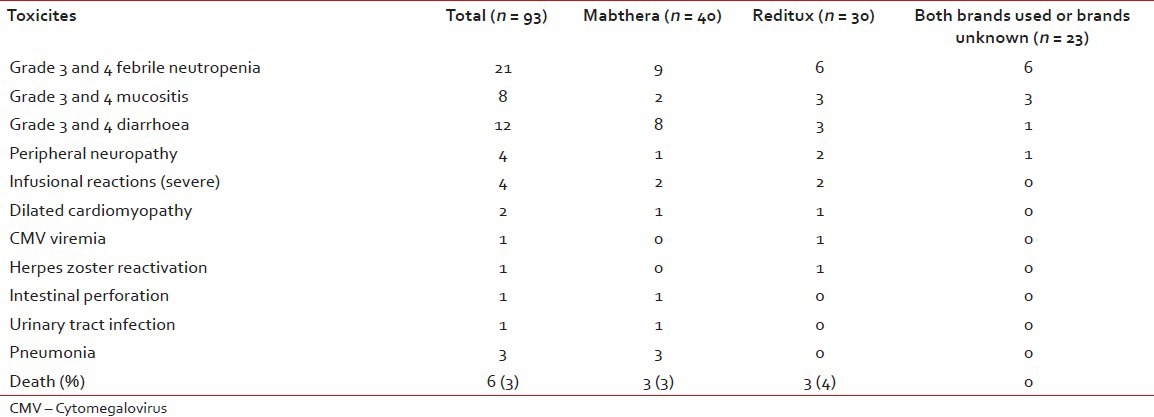
A total of 11 patients (11%) in the Mabthera™ group died, of which, 6 patients died during chemotherapy (2 died of toxicity, 2 had progressive disease and 2 of other causes) and 5 patients died during follow-up. Both the patients who died of toxicity developed grade 4 neutropenia with septicemia. Total number of deaths in Reditux™ group was 5 (7%; 1 due to toxicity (who developed septicemia), 1 of progressive disease, 1 of other cause) and 2 died during follow-up.
At last follow-up (median 30 months), 68% (151/223) patients were alive and disease free. Sixty nine percent (69/101) patients in Mabthera™ group and 77% (56/72) patients in Reditux™ group were alive and disease free.
DISCUSSION
The expected benefit of biosimilars is a reduction in acquisition expenses (cost of the drug) and consequently better access to bio therapeutics while containing the health expenditure.[10] Many Indian patients face the additional problems of lack of insurance and inaccessibility for high quality health-care. Although Mabthera™ became available in India since 2000, it was not used in most of our patients until 2007 due to the cost factor (27 patients received rituximab until 2007 and 146 patients received it post 2007). It was observed that the use of rituximab increased once biosimilars were approved for use in India.
The demographic and clinical features of DLBCL in India are similar to those from developed countries.[11] In this analysis, the CR and PR rates for all the patients were 78% and 13% respectively, PD in 4%, death during treatment was 5%; and the 5-year PFS was 68% and 5-year OS was 65%, which are comparable with randomized trials of CHOP-R (Feugier et al. in GELA LNH 98-5 and Pfreundschuh et al. in MInT trials).
In this study, analysis according to revised-IPI showed better outcomes in terms of PFS (5-year PFS in very good risk groups was 86%, in good risk group 69% and in poor risk group 49%) and OS (5-year OS in very good risk group 80%, in good risk group 61% and in poor risk group 37%).
No study has addressed the brand of rituximab used in the chemo-immunotherapy regimen. When the two brands of rituximab are compared, the responses rates, PFS, OS in the two groups were similar to the results demonstrated in various randomized trials (Feugier et al. in GELA LNH 98-5 and Pfreundschuh et al. in MInT trials). Although Reditux™ group had a better outcome in terms of 5-years PFS (81% vs. 72%; P = 0.392) and OS (76% vs. 66%; P = 0.264), it was not statistically significant. Similar results were observed when the patients receiving ≥4 cycles of the either of the brands were compared in terms of PFS (75% vs. 71% for Reditux™ vs. Mabthera™ groups, respectively) and OS (71% for Reditux™ group vs. 64% for Mabthera™ group).
Lastly, patients who received chemo-immunotherapy between 2007 and 2010, showed similar outcomes (PFS 83% and 73%; OS 78% and 72% in Reditux™ and Mabthera™ groups, respectively).
It was observed that, the two groups did not differ in the frequency of adverse events, although the toxicity data is inadequate, which is a limitation of this retrospective analysis.
In this retrospective report, the outcomes of DLBCL patients ≥18 years with the two brands of rituximab available in India are presented. Results show that Reditux™ with CHOP was equivalent to Mabthera™ with CHOP in terms of efficacy and safety.
CONCLUSION
Reditux™ is as efficacious as Mabthera™ in terms of response rates, PFS and OS with comparable toxicity. However, a randomized prospective trial is needed to validate these results.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 The number of patients treated with Mabthera™ or Reditux™ year-wise from 2004 to June 2010

| Fig. 2a The 5-year progression free survival (months) for all patients trated with cyclophosphamide, doxorubicin, vincristine and prednisolone with rituximab

| Fig. 2b 5-year progression free survival (months) for patients receiving Mabthera™ alone or Reditux™ alone in all cycles of cyclophosphamide, doxorubicin, vincristine and prednisolone with rituximab

| Fig. 3a The 5-year overall survival (months) of all patients treated with cyclophosphamide, doxorubicin, vincristine and prednisolone with rituximab

| Fig. 3b The 5-year overall survival (months) of all patients treated with Mabthera™ alone or Reditux™ alone in all cycles of cyclophosphamide, doxorubicin, vincristine and prednisolone with rituximab

| Fig. 4a The 5-year progression free survival (months) for all patients treated from 2007 to June 2010

| Fig. 4b The 5-year overall survival (months) for all patients treated from 2007 to June 2010


 PDF
PDF  Views
Views  Share
Share

