Comparison of Efficacy and Safety of Talc to Povidone-Iodine Pleurodesis in Malignant Pleural Effusion: A Systematic Review and Meta-Analysis
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(01): 024-030
DOI: 10.1055/s-0044-1796636
Abstract
Malignant pleural effusion (MPE) poses a substantial clinical challenge, necessitating effective interventions. Pleurodesis, commonly employed in MPE management, involves inducing pleural symphysis to prevent fluid accumulation. Talc and povidone-iodine have emerged as prominent agents for pleurodesis, each with its unique characteristics and considerations. This systematic review and meta-analysis aimed to compare the efficacy and safety of talc powder pleurodesis (TPP) and povidone-iodine pleurodesis (PIP) in the management of MPE. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, we conducted a systematic review registered in PROSPERO (CRD42023470930). Randomized controlled trials (RCTs) with TPP and PIP arms for MPE were included. The information sources included electronic bibliographic databases such as PubMed, Scopus, Web of Science, Cochrane, and Embase from inception to November 2023. The Cochrane risk of bias tool was used for the critical appraisal. A meta-analysis using RevMan 5.3 compared outcomes. Out of 105 identified records, 3 RCTs were included in our review. Our review findings revealed a higher success rate for TPP. However, variability existed, with one study indicating better success rates in PIP groups. Adverse events were reported less frequently in the PIP group, suggesting a potentially superior safety profile. TPP showed higher overall success in comparison to PIP, emphasizing the need for cautious clinical decision-making given variability. The potential superior safety profile of povidone-iodine underscores the importance of context-specific choices, considering patient preferences and resource constraints in selecting pleurodesis interventions for MPE management.
Keywords
malignant pleural effusion - talc pleurodesis - povidone-iodine pleurodesis - efficacy - safetyAuthors' Contributions
D.B. conceptualized the review question. D.B. and T.B. did the systematic search, data extraction, and risk of bias assessment. D.B. and T.B. interpreted the extracted data. T.B. performed the meta-analysis. D.B. and T.B. wrote the manuscript. M.K.M., S.S.M., and A.K.M. critically evaluated the manuscript. All the authors approved the final draft of the article.
Patient Consent
None declared.
Supplementary MaterialPublication History
Article published online:
29 November 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Treatment of malignant pleural effusion: consequent talc pleurodesis shows best resultsS Raab, The Thoracic and Cardiovascular Surgeon, 2010
- Pathogenesis of Malignant Pleural Effusions and Talc PleurodesisV. B. Antony, Pneumologie, 1999
- Pathogenesis of Malignant Pleural Effusions and Talc PleurodesisV. B. Antony, Pneumologie, 1999
- Pathogenesis of Malignant Pleural Effusions and Talc PleurodesisV. B. Antony, VCOT Open, 1999
- An observational study on safety and efficacy of povidone-iodine for pleurodesis in cancer patientsAyush Makkar, South Asian Journal of Cancer, 2017
- Talc pleurodesis for patients with malignant pleural effusionHiroshi Yaginuma(a), Medical*Online-E, 2018
- Efficacy and Safety of Acupuncture with Western Medicine for Rheumatoid Arthritis: A Systematic Review and Meta-analysisXinhui Huo, Acupuncture & Electro-Therapeutics Research, 2021
- The Efficacy of Recasts in Language Intervention: A Systematic Review and Meta-AnalysisPatricia L. Cleave, American Journal of Speech-Language Pathology, 2015
- Efficacy and Safety of Transcatheter Arterial Chemoembolization and Transcatheter Arterial Chemotherapy Infusion in Hepatocellular Carcinoma: A Systematic Revie...Xinyang Liu, Oncol Res, 2018
- The Effectiveness and Safety of Chemical Drugs Combined with Acupuncture for Alzheimer's Disease: A Systematic Review and Meta-AnalysisShenghua Yu, Acupuncture & Electro-Therapeutics Research, 2021
Abstract
Malignant pleural effusion (MPE) poses a substantial clinical challenge, necessitating effective interventions. Pleurodesis, commonly employed in MPE management, involves inducing pleural symphysis to prevent fluid accumulation. Talc and povidone-iodine have emerged as prominent agents for pleurodesis, each with its unique characteristics and considerations. This systematic review and meta-analysis aimed to compare the efficacy and safety of talc powder pleurodesis (TPP) and povidone-iodine pleurodesis (PIP) in the management of MPE. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, we conducted a systematic review registered in PROSPERO (CRD42023470930). Randomized controlled trials (RCTs) with TPP and PIP arms for MPE were included. The information sources included electronic bibliographic databases such as PubMed, Scopus, Web of Science, Cochrane, and Embase from inception to November 2023. The Cochrane risk of bias tool was used for the critical appraisal. A meta-analysis using RevMan 5.3 compared outcomes. Out of 105 identified records, 3 RCTs were included in our review. Our review findings revealed a higher success rate for TPP. However, variability existed, with one study indicating better success rates in PIP groups. Adverse events were reported less frequently in the PIP group, suggesting a potentially superior safety profile. TPP showed higher overall success in comparison to PIP, emphasizing the need for cautious clinical decision-making given variability. The potential superior safety profile of povidone-iodine underscores the importance of context-specific choices, considering patient preferences and resource constraints in selecting pleurodesis interventions for MPE management.
Keywords
malignant pleural effusion - talc pleurodesis - povidone-iodine pleurodesis - efficacy - safetyIntroduction
Malignant pleural effusion (MPE) poses a substantial challenge in clinical settings, requiring effective interventions to alleviate its symptoms and prevent its recurrence.[1] Pleurodesis, a common therapeutic approach, aims to induce pleural symphysis to prevent fluid accumulation due to MPE, recurrent pneumothorax, and some nonmalignant effusions. It involves chemical agents or physical abrasion during thoracotomy or thoracoscopy. The ideal agent for pleurodesis should be highly effective, having specific characteristics, yet none meet all the criteria, prompting ongoing research.[2]
Talc is widely used despite a lack of consensus on the best agent. Though effective for MPEs, concerns about talc-related acute respiratory distress syndrome arose but were later challenged.[2] Cost and availability limit medical-grade talc use, particularly in resource-poor countries. Povidone-iodine, an affordable antiseptic, proved safe and effective for pleurodesis in prior studies.[1] [2]
Talc and povidone-iodine have emerged as prominent agents for pleurodesis in MPE management due to their efficacy and safety profiles.[1] [3] Talc pleurodesis (TP), employing sterile talc powder, has long been considered a gold standard due to its high success rates in achieving symphysis and preventing effusion recurrence.[1] Povidone-iodine, an iodophor solution, has gained attention as a potential alternative to talc, this attention is attributed to povidone-iodine having higher efficacy, lower cost, and easy availability compared with talc.[1] Numerous studies have examined the efficacy, safety, and outcomes of these two agents concerning pleurodesis.[1] [3] [4] TP and povidone-iodine pleurodesis (PIP) have been evaluated for their effectiveness in managing MPE.[2] [5] The debate primarily revolves around their comparative effectiveness, safety profile, recurrence rates, and cost-effectiveness in treating MPE. Studies have compared the outcomes and safety aspects of both interventions.[1] [3] The comparison between talc and povidone-iodine in pleurodesis is crucial in resource-limited settings due to cost implications. Studies highlight povidone-iodine as a potentially cost-effective alternative to talc.[2] If both agents exhibit comparable efficacy in treating MPE, opting for povidone-iodine could be advantageous.[1] [3] Povidone-iodine's lower cost and easy availability make it appealing, especially in areas with limited resources.[1] The potential to achieve similar therapeutic outcomes while minimizing expenses might notably benefit health care systems facing constraints in funding or availability of resources.
Materials and Methods
Registration and Protocol
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting the findings.[6] The study protocol was registered in PROSPERO: International Prospective Register of Systematic Reviews (registration number: CRD42023470930) before conducting the study.
Objectives
The research question for the systematic review is “What are the comparative efficacy and safety of TP and PIP in the management of MPE?” The research question was broken down into (population, intervention, comparison, and outcome) PICO format. The defined “population” was patients with MPE, without restriction on age, sex, and ethnicity. “Intervention” was patients treated with TP, whereas “comparator” was patients treated with PIP. The “outcome” measures assessed were efficacy and safety.
Eligibility Criteria
The studies were considered eligible to be included according to the following criteria.
Inclusion Criteria
(1) Interventional studies that include both TP and PIP in MPE patients.
Exclusion Criteria
(1) Interventional studies that include a study arm involving either TP or PIP with some other comparators; Any study lacking TP and PIP interventions will be ineligible for consideration.
(2) Animal studies.
(3) Nonretrievable articles or abstract-only papers.
Information Sources and Search Strategy
We have critically reviewed the literature to select relevant articles published in the electronic bibliographic databases from inception until December 30, 2023. We systematically performed an advanced electronic search in PubMed, Scopus, Web of Science, Cochrane, and Embase for eligible studies. The search strategy in the above database was performed using the keywords and Medical Subject Headings (MeSH) terms like “malignant pleural effusion,” “Pleurodesis,” “povidone iodine,” “talc,” using “AND” and “OR” ([Supplementary File S1], available in the online version). We limited the search to English publications.
Study Selection Process and Data Extraction
The studies were screened by title and abstracts followed by full-text articles based on predefined criteria. Two independent reviewers (D.B. and T.B.) performed the study selection, and disagreements were resolved by mutual consultation with a third reviewer (M.K.M). A well-defined data extraction sheet was employed for data extraction. Data from the final selected studies included authors' names, year of publication, study design, sample size, study groups, clinical outcomes, and adverse effects. One reviewer (T.B.) extracted the data in a standardized extraction sheet, and the other reviewer (D.B.) checked for accuracy. Any disagreement was resolved by a mutual discussion or consultation with a third reviewer (M.K.M).
Risk of Bias Assessment
The Cochrane risk of bias assessment tool was used to assess the methodological quality of the included studies.[7] Two independent reviewers (D.B. and T.B.) performed the quality assessment, and any disagreements between the reviewers were settled through consensus or discussion with a third reviewer (M.K.M.).
Data Synthesis
A narrative synthesis was performed from the extracted data findings and presented in tabular form. The data synthesized in the review summarized the current evidence of efficacy and safety for TP and PIP in MPE. Subgroup analysis could not be performed due to insufficient available data.
Statistical Analysis
We used the Review Manager software (RevMan, version 5.3 for Windows; The Cochrane Collaboration, Oxford, United Kingdom) to conduct a meta-analysis, and odds ratio (OR) with 95%- confidence interval (CI) values was calculated.[8] Statistical heterogeneity of data was assessed using the I 2 statistic, and the fixed-effects model was used for studies without significant heterogeneity (I 2 ≤ 50%- or p ≥ 0.10). We could not create a funnel plot for assessing visual inspection of publication bias due to a lack of sufficient eligible studies.
Results
Study Selection
We identified 105 records by searching the MeSH terms from the abovementioned databases. We removed 54 duplicate records before the screening, leaving 51 unique records for further assessment. Out of these, 43 records were excluded, indicating the stringent application of predefined inclusion/exclusion criteria. Subsequently, efforts were made to retrieve eight reports for closer evaluation, although only six reports were successfully obtained and assessed for eligibility. Within this assessment, four reports were excluded, comprising two conference abstracts, one trial protocol, and one observational study that did not meet the desired comparator criteria. We employed a manual search strategy that resulted in the identification of two records. Subsequently, reports sought for retrieval and reports assessed for eligibility both amounted to two records each. However, one report was excluded due to being in a language other than the specified language criterion. Finally, we incorporated a total of three studies in the review.
Study Characteristics
All the final selected studies were randomized controlled trials (RCTs). We identified all research papers by the first author's last name and year ([Table 1]). In tabular format, we recorded the extracted data from the three studies.[1] [3] [4] Among the included studies, two were conducted in Egypt[1] [3] and one was conducted in India.[4] We extracted data from a study group consisting of patients with MPE who underwent pleurodesis with either povidone-iodine or talc. The flowchart for study selection according to the PRISMA guidelines is shown in [Fig. 1]. Among the three included studies, Agarwal et al had conducted their study by taking either benign or MPE.[4] Hence, according to our review criteria, we have extracted data about MPE only.
|
Author/Year Ref |
Regions |
Study design |
Study duration |
Population |
|---|---|---|---|---|
|
Ibrahim et al, 2015[1] |
Egypt |
RCT |
Not available |
Recurrent MPE |
|
Mohsen et al, 2011[3] |
Egypt |
RCT |
January 2002 to December 2005 |
MPE |
|
Agarwal et al, 2011[4] |
India |
RCT |
January 2006 to June 2007 |
MPE |
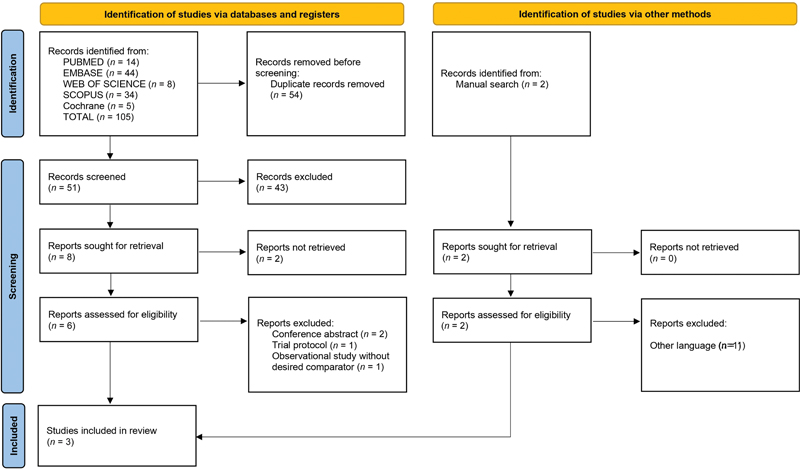
| Fig 1 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the screening and selection process.|
Overall Efficacy
To compare the efficacy of TP and PIP procedures, we identified and extracted the data on outcome measures, which were in terms of success rate, complete inflation, partial inflation, and failure rate ([Table 2]).
|
Study |
Number of participants |
Success rate |
Complete inflation |
Partial inflation |
Failure |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Talc, n |
Povidone-iodine, n |
Talc, n (%) |
Povidone-iodine, n (%) |
Talc, n (%) |
Povidone-iodine, n (%) |
Talc, n (%) |
Povidone-iodine, n (%) |
Talc, n (%) |
Povidone-iodine, n (%) |
|
|
Ibrahim et al, 2015[1] |
21 |
18 |
17 (80.85) |
13 (72.22) |
15 (71.43) |
12 (66.66) |
02 (9.52) |
01 (5.55) |
04 (19.04) |
05 (27.77) |
|
Mohsen et al, 2011[3] |
22 |
20 |
20 (90.90) |
17 (85%) |
19 (86.36%) |
17 (85%) |
01 (4.54%) |
00 (0%) |
02 (9.09) |
03 (15) |
|
Agarwal et al, 2011[4] |
18 |
18 |
16[a] (90) |
17[a] (95) |
. |
. |
. |
. |
. |
. |
|
StudyRef |
Pain |
Fever |
Recurrence of dyspnea |
Allergy |
Death |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Talc, n (%) |
Povidone-iodine, n (%) |
Talc, n (%) |
Povidone-iodine, n (%) |
Talc, n (%) |
Povidone-iodine, n (%) |
Talc, n (%) |
Povidone-iodine, n (%) |
Talc, n (%) |
Povidone-iodine, n (%) |
|
|
Ibrahim et al, 2015[1] |
14 (66.66) |
09 (50) |
04 (19.04) |
04 (22.22) |
04 (19.04) |
05 (27.77) |
00 (0) |
00 (0) |
00 (0) |
00 (0) |
|
Mohsen et al, 2011[3] |
04 (18.18) |
00 (0) |
04 (18.18) |
01 (5) |
00 (0) |
. |
. |
. |
. |
. |
|
Agarwal et al, 2011[4] |
. |
. |
. |
. |
. |
. |
. |
. |
. |
. |
|
StudiesRef |
Sequence generation |
Allocation concealment |
Incomplete outcome data |
Selective reporting |
Other bias |
|---|---|---|---|---|---|
|
Ibrahim et al, 2015[1] |
Low |
High |
Low |
Low |
Low |
|
Mohsen et al, 2011[3] |
Low |
Low |
Low |
Low |
Low |
|
Agarwal et al, 2011[4] |
Low |
Low |
Low |
Low |
Low |
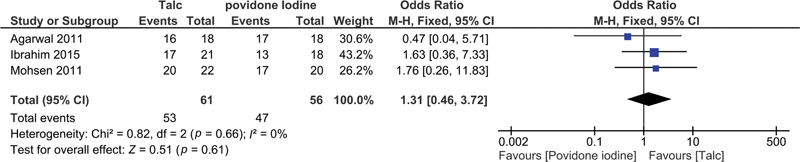
| Fig 2 The success rate in talc pleurodesis versus povidone-iodine pleurodesis.|
Discussion
Our systematic review aimed to compare the efficacy and safety of TP versus PIP in managing MPE. We found that TP demonstrated a higher success rate in achieving pleurodesis than PIP.[1] [3] However, one of the studies included in the analysis presented differing results, leading to variability in findings.[4] While both interventions were generally well-tolerated, adverse events like pain and fever were reported less frequently in the povidone-iodine group, suggesting a superior safety profile for povidone-iodine. The clinical importance of these findings lies in the similar outcomes of TP and PIP in providing effective and sustained relief for patients with MPE. However, it is essential to consider the small sample size in all the included studies, which may impact the generalizability of these results. A retrospective observational study by Makkar et al showed a 79%- success rate in pleurodesis with manageable pain and minimal complications.[9] In a meta-analysis, Muthu et al suggested that povidone-iodine is a cost-effective and widely available alternative, with a success rate of approximately 90%.[10] Their findings revealed no notable adverse effects like thyroid dysfunction or iodine toxicity, at standard doses. While talc poudrage may be superior according to a network meta-analysis, tube thoracostomy with povidone-iodine remains a practical option, especially in resource-constrained settings.[11] Another meta-analysis by Beltsios et al noted the potential superiority of TP over other mechanical approaches.[5] Comparatively better success rate associated with TP suggests that it may be a preferred option for more robust and sustained pleurodesis in patients with MPE. Clinicians may consider TP as a first-line intervention, particularly in cases where long-term efficacy is a primary concern. However, the superior safety profile of PIP, with fewer reported adverse events, presents an important alternative, especially in situations where minimizing complications is a priority. Clinicians should weigh the potential benefits of effective pleurodesis against the safety considerations when selecting the appropriate intervention for individual patients. Our findings align with the study by Muthu et al supporting the role of povidone-iodine as a cost-effective and widely available alternative with a pooled success rate of 90%.[10] A recent comprehensive review conducted by Bonser et al concluded that the current standard of care for pleurodesis in MPE is based on limited evidence.[12]
The study limitations hinder the generalizability of recommendations. However, the available evidence suggests that povidone-iodine is a safe, well-tolerated, and equally effective agent for achieving palliative pleurodesis in MPE. Povidone-iodine has several advantages including low cost, accessibility, and ease of administration, making it a suitable alternative to talc in certain clinical settings.[12] In resource-constrained settings, where talc may pose logistical challenges, povidone-iodine emerges as a practical option without compromising efficacy. Clinicians must consider the local context, patient preferences, and available resources when making decisions about pleurodesis interventions. While TP may be favored in settings with adequate resources and expertise, PIP can be a valuable alternative in situations where logistical constraints or safety concerns are paramount. The choice between TP and PIP should be made through shared decision-making involving the patient, considering individual factors such as comorbidities, treatment goals, and preferences. Additionally, ongoing monitoring for adverse events, patient response, and the need for repeat procedures should be integral components of post-pleurodesis care, irrespective of the chosen intervention. This nuanced understanding of the comparative effectiveness and safety profiles of TP and PIP should inform evidence-based decision-making in the clinical management of MPE. However, this systematic review has certain limitations. The primary constraint lies in the small sample size of the included studies, which may limit the generalizability of the findings. Additionally, variations in, the type of malignancy, and follow-up durations across the three RCTs might have influenced the heterogeneity. Also, including studies reporting no significant statistical differences between TP and PIP could have influenced the pooled results. However, their inclusion was necessary to reduce publication bias and provide a more balanced and comprehensive analysis of the available studies on TP versus PIP in MPE patients. These warrant need for a larger, greater number of studies to strengthen the evidence base for informed clinical decision-making.
Future Directions
Future research should concentrate on conducting well-designed multicenter RCTs with larger sample sizes. Standardization of study protocols, including consistent outcome measures, will enhance the comparability of results across trials. Additionally, exploring patient-specific factors that may influence the choice of pleurodesis agent and conducting long-term follow-up studies are critical steps in advancing our understanding of the comparative effectiveness of TP and PIP. These may help to develop a prediction model for point care for MPE.
Conclusion
Although TP is more effective in achieving pleurodesis, the PIP treatment appears to have a better safety profile with fewer adverse effects. The importance of these results is that both treatments provide effective and long-lasting relief for patients with MPE. However, further research is needed, including larger and more standardized trials, to build on these findings and refine treatment guidelines in this clinical context.
Conflict of Interest
None declared.
Acknowledgments
None.
Authors' Contributions
D.B. conceptualized the review question. D.B. and T.B. did the systematic search, data extraction, and risk of bias assessment. D.B. and T.B. interpreted the extracted data. T.B. performed the meta-analysis. D.B. and T.B. wrote the manuscript. M.K.M., S.S.M., and A.K.M. critically evaluated the manuscript. All the authors approved the final draft of the article.
Patient Consent
None declared.
Supplementary MaterialReferences
- Ibrahim IM, Dokhan AL, El-Sessy AA, Eltaweel MF. Povidone-iodine pleurodesis versus talc pleurodesis in preventing recurrence of malignant pleural effusion. J Cardiothorac Surg 2015; 10: 64
- Agarwal R, Khan A, Aggarwal AN, Gupta D. Efficacy & safety of iodopovidone pleurodesis: a systematic review & meta-analysis. Indian J Med Res 2012; 135 (03) 297-304
- Mohsen TA, Zeid AA, Meshref M. et al. Local iodine pleurodesis versus thoracoscopic talc insufflation in recurrent malignant pleural effusion: a prospective randomized control trial. Eur J Cardiothorac Surg 2011; 40 (02) 282-286
- Agarwal R, Paul AS, Aggarwal AN, Gupta D, Jindal SK. A randomized controlled trial of the efficacy of cosmetic talc compared with iodopovidone for chemical pleurodesis. Respirology 2011; 16 (07) 1064-1069
- Beltsios ET, Mavrovounis G, Adamou A, Panagiotopoulos N. Talc pleurodesis in malignant pleural effusion: a systematic review and meta-analysis. Gen Thorac Cardiovasc Surg 2021; 69 (05) 832-842
- Page MJ, McKenzie JE, Bossuyt PM. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372 (71) n71
- Higgins JP, Altman DG, Gøtzsche PC. et al; Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928
- Higgins J, Thomas J. Online version 6.4. Cochrane Handbook for Systematic Reviews of Interventions, Cochrane Training; 2023: Section 10–6
- Makkar A, Patni S, Joad AK, Lakhera KK. An observational study on safety and efficacy of povidone-iodine for pleurodesis in cancer patients. South Asian J Cancer 2017; 6 (02) 79-80
- > Muthu V, Dhooria S, Sehgal IS, Prasad KT, Aggarwal AN, Agarwal R. Iodopovidone pleurodesis for malignant pleural effusions: an updated systematic review and meta-analysis. Support Care Cancer 2021; 29 (08) 4733-4742
- > Dipper A, Jones HE, Bhatnagar R, Preston NJ, Maskell N, Clive AO. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev 2020; 4 (04) CD010529
- > Bonser SA, Zhu MZL, McKay GS. Is povidone-iodine pleurodesis as effective, safe and well tolerated as talc pleurodesis for recurrent malignant pleural effusions?. Interdiscip Cardiovasc Thorac Surg 2024; 38 (01) ivad192
Address for correspondence
Mohan K. Manu, MBBS, DNBDepartment of Respiratory Medicine, Kasturba Medical College, Manipal, Manipal Academy of Higher EducationManipal 576104, KarnatakaIndiaEmail: manu.mohan@manipal.eduPublication History
Article published online:
29 November 2024© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, IndiaWe recommend- Treatment of malignant pleural effusion: consequent talc pleurodesis shows best resultsS Raab, The Thoracic and Cardiovascular Surgeon, 2010
- Pathogenesis of Malignant Pleural Effusions and Talc PleurodesisV. B. Antony, Pneumologie, 1999
- Pathogenesis of Malignant Pleural Effusions and Talc PleurodesisV. B. Antony, Pneumologie, 1999
- Pathogenesis of Malignant Pleural Effusions and Talc PleurodesisV. B. Antony, VCOT Open, 1999
- An observational study on safety and efficacy of povidone-iodine for pleurodesis in cancer patientsAyush Makkar, South Asian Journal of Cancer, 2017
- Is povidone-iodine pleurodesis as effective, safe and well tolerated as talc pleurodesis for recurrent malignant pleural effusions?Sophie A Bonser, Interactive CardioVascular and Thoracic Surgery
- Indwelling Pleural Catheter versus Pleurodesis for Malignant Pleural Effusions: A Systematic Review and Meta-AnalysisNarayan P Iyer, Ann Am Thorac Soc, 2018
- Rapid pleurodesis in symptomatic malignant pleural effusionErkan Yildirim, European Journal of Cardio-Thoracic Surgery, 2005
- Matrix metalloproteinases production in malignant pleural effusions after talc pleurodesisP DAGOSTINO, Clinical and Experimental Immunology, 2003
- Long-term follow-up of video-assisted talc pleurodesis in malignant recurrent pleural effusionsG. Cardillo, European Journal of Cardio-Thoracic Surgery, 2002
- Treatment of malignant pleural effusion: consequent talc pleurodesis shows best results

| Fig 1 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the screening and selection process.|

| Fig 2 The success rate in talc pleurodesis versus povidone-iodine pleurodesis.|
References
- Ibrahim IM, Dokhan AL, El-Sessy AA, Eltaweel MF. Povidone-iodine pleurodesis versus talc pleurodesis in preventing recurrence of malignant pleural effusion. J Cardiothorac Surg 2015; 10: 64
- Agarwal R, Khan A, Aggarwal AN, Gupta D. Efficacy & safety of iodopovidone pleurodesis: a systematic review & meta-analysis. Indian J Med Res 2012; 135 (03) 297-304
- Mohsen TA, Zeid AA, Meshref M. et al. Local iodine pleurodesis versus thoracoscopic talc insufflation in recurrent malignant pleural effusion: a prospective randomized control trial. Eur J Cardiothorac Surg 2011; 40 (02) 282-286
- Agarwal R, Paul AS, Aggarwal AN, Gupta D, Jindal SK. A randomized controlled trial of the efficacy of cosmetic talc compared with iodopovidone for chemical pleurodesis. Respirology 2011; 16 (07) 1064-1069
- Beltsios ET, Mavrovounis G, Adamou A, Panagiotopoulos N. Talc pleurodesis in malignant pleural effusion: a systematic review and meta-analysis. Gen Thorac Cardiovasc Surg 2021; 69 (05) 832-842
- Page MJ, McKenzie JE, Bossuyt PM. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372 (71) n71
- Higgins JP, Altman DG, Gøtzsche PC. et al; Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928
- Higgins J, Thomas J. Online version 6.4. Cochrane Handbook for Systematic Reviews of Interventions, Cochrane Training; 2023: Section 10–6
- Makkar A, Patni S, Joad AK, Lakhera KK. An observational study on safety and efficacy of povidone-iodine for pleurodesis in cancer patients. South Asian J Cancer 2017; 6 (02) 79-80
- > Muthu V, Dhooria S, Sehgal IS, Prasad KT, Aggarwal AN, Agarwal R. Iodopovidone pleurodesis for malignant pleural effusions: an updated systematic review and meta-analysis. Support Care Cancer 2021; 29 (08) 4733-4742
- > Dipper A, Jones HE, Bhatnagar R, Preston NJ, Maskell N, Clive AO. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev 2020; 4 (04) CD010529
- > Bonser SA, Zhu MZL, McKay GS. Is povidone-iodine pleurodesis as effective, safe and well tolerated as talc pleurodesis for recurrent malignant pleural effusions?. Interdiscip Cardiovasc Thorac Surg 2024; 38 (01) ivad192


 PDF
PDF  Views
Views  Share
Share

