Clinicopathological Features and Outcomes in Primary Central Nervous System Lymphoma: A 10 year Experience
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 478-482
DOI: DOI: 10.4103/ijmpo.ijmpo_202_16
Abstract
Context: Primary central nervous system lymphoma (PCNSL) is a variant of extranodal lymphoma, accounting for 4% of primary central nervous system tumors. PCNSL was morecommon in immunocompetent individuals. International Extranodal Lymphoma Study Group (IELSG) scoring was used for prognostication. High-dose methotrexate regimens along with radiotherapy improved outcomes in PCNSL. Aims: The aim of this study is to analyze the clinical and pathological features, progression-free survival (PFS), and overall survival (OS) in patients with PCNSL. Materials and Methods: Data of patients with PCNSL between 2005 and 2016 were retrospectively analyzed. Outcome was analyzed in patients who received chemotherapy. GraphPad Prism software for Windows Version 6 was used to plot the Kaplan–Meier curves for PFS and OS. Log-rank test was used to calculate P values. P < 0 class="b" xss=removed>Results: A total of 42 patients were available for analysis. Of these, 34 patients who received chemotherapy were evaluable for outcome parameters. The median age at presentation was 46 years (range, 10–75) with male-to-female ratio of 2.2:1. Only 2 (4.7%) patients were HIV positive. Diffuse large B-cell lymphoma (DLBCL) was the mostcommon histology seen in 41 (97.6%) patients. Using IELSG risk scoring, scores of 8 (19%), 19 (45.2%), and 15 (35.8%) were stratified into low, intermediate, and high risk. The median PFS and OS were 11 months (range, 2–72) and 15.9 months (2.4–80.4), respectively. The median OS was 36.2 months (range, 8.8–72), 15.6 months (2–36), and 6.1 months (2.6–12.7) in low-, intermediate-, and high-risk groups, respectively, which was statistically significant (P = 0.0002). Conclusions: Immunocompetent patients with PCNSL outnumber immunocompromised patients. DLBCL was the mostcommon histology, and IELSG risk stratification significantly predicts the outcome in PCNSL.
Keywords
International Extranodal Lymphoma Study Group - primary central nervous system lymphoma - survivalPublication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
Primary central nervous system lymphoma (PCNSL) is a variant of extranodal lymphoma, accounting for 4% of primary central nervous system tumors. PCNSL was more common in immunocompetent individuals. International Extranodal Lymphoma Study Group (IELSG) scoring was used for prognostication. High-dose methotrexate regimens along with radiotherapy improved outcomes in PCNSL.
Aims:
The aim of this study is to analyze the clinical and pathological features, progression-free survival (PFS), and overall survival (OS) in patients with PCNSL.
Materials and Methods:
Data of patients with PCNSL between 2005 and 2016 were retrospectively analyzed. Outcome was analyzed in patients who received chemotherapy. GraphPad Prism software for Windows Version 6 was used to plot the Kaplan–Meier curves for PFS and OS. Log-rank test was used to calculate P values. P < 0>
Results:
A total of 42 patients were available for analysis. Of these, 34 patients who received chemotherapy were evaluable for outcome parameters. The median age at presentation was 46 years (range, 10–75) with male-to-female ratio of 2.2:1. Only 2 (4.7%) patients were HIV positive. Diffuse large B-cell lymphoma (DLBCL) was the most common histology seen in 41 (97.6%) patients. Using IELSG risk scoring, scores of 8 (19%), 19 (45.2%), and 15 (35.8%) were stratified into low, intermediate, and high risk. The median PFS and OS were 11 months (range, 2–72) and 15.9 months (2.4–80.4), respectively. The median OS was 36.2 months (range, 8.8–72), 15.6 months (2–36), and 6.1 months (2.6–12.7) in low-, intermediate-, and high-risk groups, respectively, which was statistically significant (P = 0.0002).
Conclusions:
Immunocompetent patients with PCNSL outnumber immunocompromised patients. DLBCL was the most common histology, and IELSG risk stratification significantly predicts the outcome in PCNSL.
Introduction
Primary central nervous system lymphoma (PCNSL) is an uncommon variant of extranodal non-Hodgkin's lymphoma (NHL) involving brain, leptomeninges, eyes, or spinal cord without evidence of systemic disease. PCNSL represents approximately 4% of primary central nervous system tumors and 1% of all intracranial neoplasms.[1,2,3] Histologically, most of the PCNSLs were diffuse large B-cell lymphoma (DLBCL). Other histologies were seen in very few cases.[4,5,6,7] Most were immunocompetent individuals.[7,8] Usage of high-dose methotrexate-based chemotherapeutic regimens with or without whole-brain radiotherapy had improved survivals in PCNSL.[9,10,11] Addition of rituximab had further improved survivals in PCNSL, and the International Extranodal Lymphoma Study Group (IELSG) prognostic score has shown to be a useful predictor of survival in PCNSL patients.[12,13] The primary objectives of this analysis were to study the clinicopathological and outcomes in PCNSL patients.
Materials and Methods
A contrast enhanced magnetic resonance imaging (MRI) of brain, Ophthalmological examination with slit lamp and fundoscopy were performed in all patients. Contrast-enhanced computed tomography (CECT) scan of the neck, chest, abdomen, and pelvis or positron emission tomography (PET)-CT scan, bone marrow aspiration and biopsy, testicular ultrasound examination, and cerebrospinal fluid analysis were performed to exclude systemic lymphoma. The diagnosis was confirmed by stereotactic biopsy or excision of brain lesion.
Blood investigations included a complete hemogram, serum lactate dehydrogenase (LDH), liver function tests, and renal function tests. The IELSG prognostic score[14] was calculated for each patient based on five characteristics, i.e., age, Eastern Cooperative Oncology Group Performance Status, deep brain structure involvement, cerebrospinal fluid (CSF) protein elevation, and serum LDH levels. Each variable was assigned a value of 0 if favorable or of 1 if unfavorable, and the values of the five variables were added for final score calculation. Patients were grouped into risk groups based on final score as low risk (score 0–1), intermediate risk (score 2–3), and high risk (score 4–5).
Progression-free survival (PFS) was defined as the time from start of chemotherapy to the time that PD was documented, death, or lost to follow-up. Overall survival (OS) was defined as the time from start of chemotherapy to death due to any cause or lost to follow-up.
GraphPad Prism software for Windows Version 6 was used to plot the Kaplan–Meier curves for PFS and OS (GraphPad Software, La Jolla, California, USA, www.graphpad.com). Log-rank test was used to calculate P values. P < 0>
Results
Forty-two patients who were diagnosed with PCNSL between 2006 and 2016 were retrospectively analyzed. The median age at presentation was 46 years (range, 10–75) with male-to-female ratio of 2.2:1. The baseline characteristics of all patients were shown in Table 1. Only 2 patients were HIV positive and rest all were HIV negative.
Table 1
Demographic features
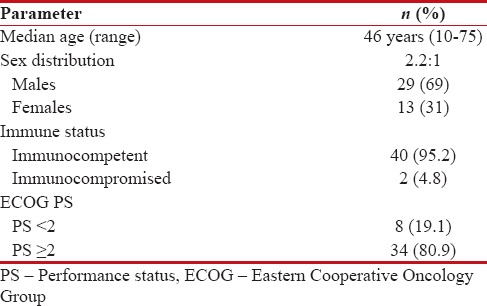
|
Clinicopathological features
The most common symptom at presentation was headache (64.2%), followed by neurological deficits (54.7%), vomiting (47.6%), and neuropsychiatric features (19%), respectively. Diagnosis of PCNSL was based on stereotactic biopsy and excisional biopsy in 18 (42.8%) and 24 (57.1%), respectively. DLBCL was the most common histology seen in 41 (97.6%) patients, and Burkitt lymphoma was the histology in 1 (2.4%) patient. All patients were Leukocyte Common Antigen (LCA) and CD20 positive and CD3 negative. The clinicopathological features of all patients are depicted in Table 2.
Table 2
Clinicopathological features (n=42)
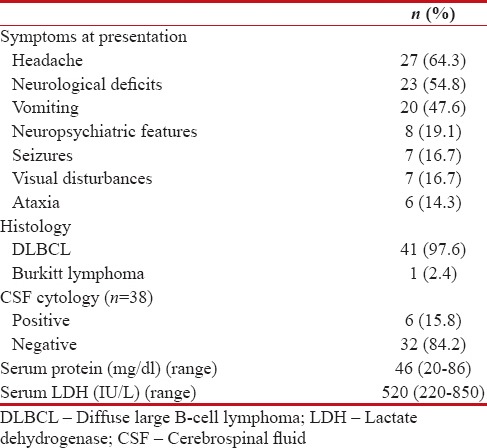
| The site and focality of the lesions in MRI scan were shown in Table 3. PET-CT scan was done in 10 (23.8%) patients, and CECT of the neck, chest, abdomen, and pelvis was done in 32 (76.2%) patients to exclude systemic lymphoma. CSF cytology was done in 38 patients, and out of which, 6 (15.8%) patients had positive CSF cytology and 32 (84.2%) patients had negative CSF cytology. CSF cytology was not done in 4 patients.
Table 3
Site and focality of the lesion (n=42)

|
Risk stratification
According to IELSG risk stratification, 8 (19%), 19 (45.2%), and 15 (35.8%) patients were stratified into low, intermediate, and high risk, respectively. Risk stratification according to IELSG risk score was shown in Table 4.
Table 4
International Extranodal Lymphoma Study Group risk stratification

|
Treatment outcomes for all patients
A total of 34 (80.9%) received chemotherapy with or without radiotherapy. Eight (19.1%) patients defaulted and not received any treatment.
The treatment regimens used were shown in Table 5. DeAngelis protocol was the most common treatment regimen used (27/34, 79.4%). Rituximab was added to DeAngelis in 29.4% of patients. The other regimens used were steroid with radiotherapy and Berlin–Frankfurt–Munster-NHL protocol in 6 (17.7%) and 1 (2.9%) patients, respectively.
Table 5
Treatment received (n=34)

|
With a median follow-up period was 18 months, the median PFS and OS were 11 months (range, 2–72) and 15.9 months (range, 2.4–80.4), respectively. The 1-year, 2-year, and 3-year survivals were 61.7%, 32.3%, and 17.6%, respectively. The median PFS and OS were shown in Figures Figures11 and and2.2. The median PFS and OS for patients taking DeAngelis protocol were 12.7 months (range, 2–72) and 18.6 months (range, 2.4–80.4), respectively.
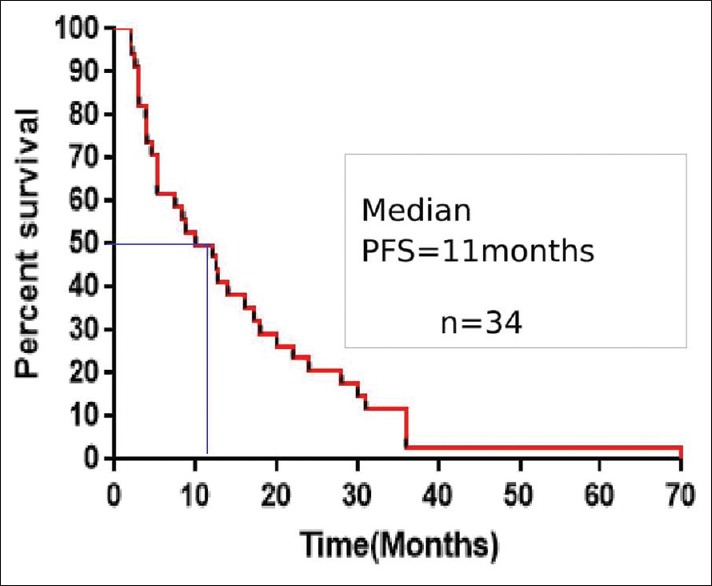
| Figure 1:Kaplan–Meier estimates of progression-free survival for all patients (PFS – Progression-free survival)
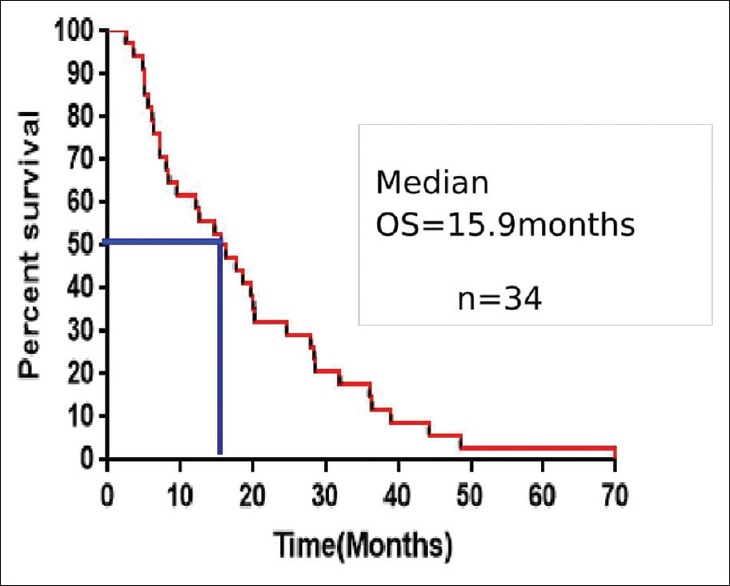
| Figure 2:Kaplan–Mein curve comparing the survival between males and femalesKaplan–Meier estimates of overall survival for all patients (OS – Overall survival)
The median PFS in low, intermediate, and high risk was 30.5 months (range, 8.8–72), 12 months (range, 2–36), and 4.5 months (range, 2.6–12.7), respectively (P ≤ 0.0001). The median OS was 36.2 months (range, 14.7–80.4), 15.6 months (range, 4.8–39), and 6.1 months (range, 2.4–15.1) in low-, intermediate-, and high-risk patients, respectively (P = 0.0002). The median PFS and OS according to IELSG risk scoring was shown in Figures Figures33 and and4,4, respectively.
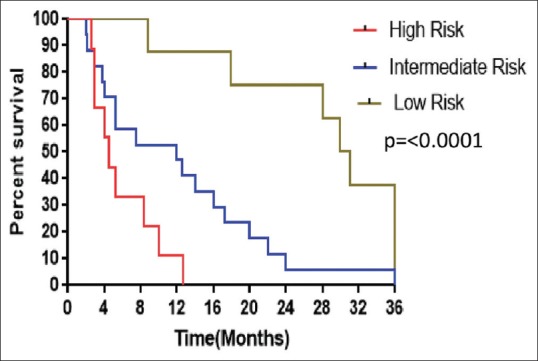
| Figure 3:Kaplan–Meier estimates of progression-free survival of patients based on International Extranodal Lymphoma Study Group risk scoring
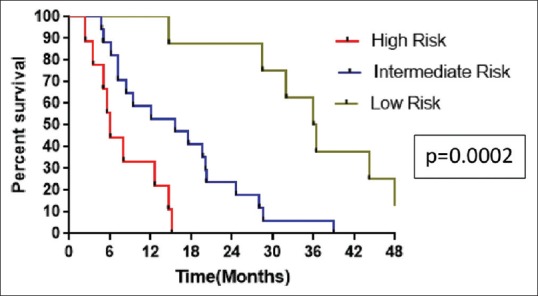
| Figure 4:Kaplan–Meier estimates of overall survival of patients based on International Extranodal Lymphoma Study Group risk scoring
Discussion
This is a retrospective analysis of patients with PCNSL. In our study, we had evaluated all patients who were diagnosed as primary central nervous lymphoma and those who received chemotherapy and radiotherapy were evaluated for outcome parameters.
Of the 42 patients, 34 were evaluable for outcome parameters. Eight patients were lost to follow-up after diagnosis. They were excluded from the analysis of outcome parameters.
The median age at presentation of 46 years was similar to other studies by Lakshmaiah et al.,[15] Paul et al.,[16] and Pasricha et al.[17] but lower than compared to studies by Haldorsen et al.,[18] Agarwal et al.,[19] and Gupta et al.[20] The gender ratio of 2.2:1 was higher compared to other Indian studies. The comparison of demographic and pathological features with other studies was tabulated in Table 6.
Table 6
Comparison of demographic and clinicopathological features with other studies
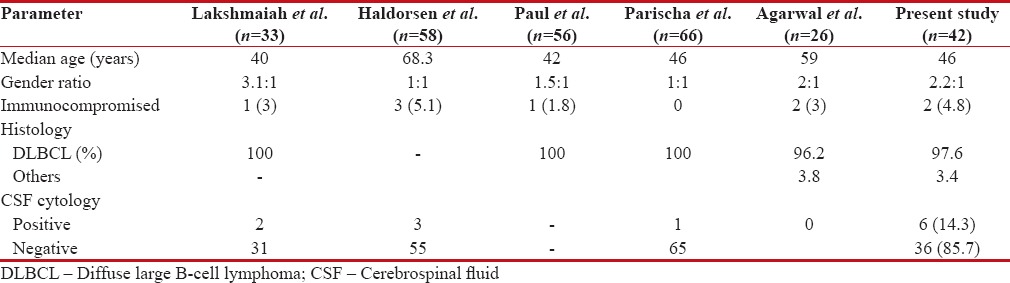
| DLBCL was the most common histology accounting for 97.6% of cases similar to the studies by Lakshmaiah et al., Paul et al., Pasricha et al., and Agarwal et al., where 95%–100% of cases were DLBCL. On imaging, solitary lesions are generally considered more common than multiple lesions. In this analysis, solitary lesions were higher than multiple lesions, similar to studies by Gupta et al., Pasricha et al., and Paul et al. but in contrast to studies by Agarwal et al. and Herrlinger et al.[21] which showed multiple lesions are more common than solitary lesions.
The incidence of CSF cytology positivity in immunocompetent patients is reported be 26%–31%.[22] However, in the present study, only 14% had cytology positivity. This was higher than the reports by Agarwal et al. and Lakshmaiah et al. and similar to that reported by Haldorsen et al.
Various malignant and nonmalignant conditions such as gliomas, metastases, toxoplasmosis, tuberculosis, and progressive multifocal leukoencephalopathy are differential diagnosis for PCNSL, and it is not possible to diagnose PCNSL radiologically in all cases; hence, histopathological examination is mandatory for confirmation of diagnosis. In the present study, 42.8% and 57.2% had diagnosis based on stereotactic biopsy and surgical excision, respectively. CECT of the neck, chest, abdomen, and pelvis or PET-CT scan should be used to exclude disease outside the central nervous system in all cases of PCNSL. In the present study, majority of the patients had CECT scan and only 19% had PET-CT scan as a part of routine staging workup.
IELSG and MSKCC scoring systems[23] are used to prognosticate patients in PCNSL. In the present study, IELSG scoring system was used to risk stratify patients into low, intermediate, and high risk. Treatment of PCNSL involves a multimodality approach with chemotherapy and radiotherapy. With the advent of high-dose methotrexate-based regimens, survivals in PCNSL were improved dramatically and now they became the standard of care. The addition of rituximab to high-dose methotrexate further improved the survivals in PCNSL. In the present study, 50% and 33.3% of patients received high-dose methotrexate chemotherapy with and without rituximab, respectively. Eight patients were lost to follow-up due to various reasons and had not received any further therapy.
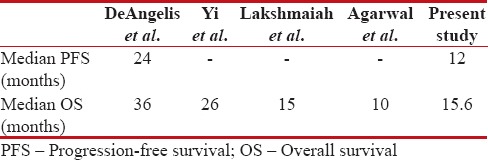
In a large study by DeAngelis et al.[24] with 102 patients, the use of high-dose methotrexate, high-dose cytarabine, and whole-brain radiotherapy resulted in median PFS of 24 months and OS of 36 months. Abrey et al.[25] in their study on long-term survival in PCNSL patients had shown a median cause-specific survival of 42 months, and Yi et al.[26] in their study showed median OS of 26 months with DeAngelis protocol. The median OS of 15.6 months in the present study was similar to study by Lakshmaiah et al. but higher than the study by Agarwal et al.
Risk stratification according to IELSG risk scoring done in the present study showed the median OS of 36.2 months, 15.6 months, and 6.1 months in low-, intermediate-, and high-risk groups, respectively, showing that IELSG risk scoring had a significant impact on outcome. The comparison of survivals with other studies is tabulated in Table 7.
Table 7
Comparison of survival with other studies
| The drawbacks of the present study are that it is retrospective, with limited data on adverse effects of chemotherapy. There is an incomplete documentation of hematological and nonhematological toxicity.
Conclusions
The findings of this study have significant implications for clinical practice. Immunocompetent patients with PCNSL outnumber immunocompromised patients. DLBCL is the most common histology of PCNSL. Even though survivals had improved with high-dose methotrexate regimens, they are still low compared to lymphomas of other sites. There is a need to develop more effective regimens. IELSG risk scoring system had a significant impact on survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985-1999. Neuro Oncol 2006;8:27-37.
- Shah GD, DeAngelis LM. Treatment of primary central nervous system lymphoma. Hematol Oncol Clin North Am 2005;19:611-27, v.
- Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG, et al. Descriptive epidemiology of primary brain and CNS tumors: Results from the central brain tumor registry of the United States, 1990-1994. Neuro Oncol 1999;1:14-25.
- Miller DC, Hochberg FH, Harris NL, Gruber ML, Louis DN, Cohen H, et al. Pathology with clinical correlations of primary central nervous system non-hodgkin's lymphoma. The Massachusetts general hospital experience 1958-1989. Cancer 1994;74:1383-97.
- Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg 1988;68:835-53.
- Krogh-Jensen M, D'Amore F, Jensen MK, Christensen BE, Thorling K, Pedersen M, et al. Clinicopathological features, survival and prognostic factors of primary central nervous system lymphomas: Trends in incidence of primary central nervous system lymphomas and primary malignant brain tumors in a well-defined geographical area. Population-based data from the Danish Lymphoma Registry, LYFO, and the Danish Cancer Registry. Leuk Lymphoma 1995;19:223-33.
- Fine HA, Mayer RJ. Primary central nervous system lymphoma. Ann Intern Med 1993;119:1093-104.
- Rubenstein JL, Treseler P, O'Brien JM. Pathology and genetics of primary central nervous system and intraocular lymphoma. Hematol Oncol Clin North Am 2005;19:705-17, vii.
- Ferreri AJ, Reni M, Villa E. Therapeutic management of primary central nervous system lymphoma: Lessons from prospective trials. Ann Oncol 2000;11:927-37.
- Ferreri AJ, Reni M, Pasini F, Calderoni A, Tirelli U, Pivnik A, et al. Amulticenter study of treatment of primary CNS lymphoma. Neurology 2002;58:1513-20.
- ;Joerger M, Huitema AD, Krähenbühl S, Schellens JH, Cerny T, Reni M, et al. Methotrexate area under the curve is an important outcome predictor in patients with primary CNS lymphoma: A pharmacokinetic-pharmacodynamic analysis from the IELSG no 20 trial. Br J Cancer 2010;102:673-7.
- Gregory G, Arumugaswamy A, Leung T, Chan KL, Abikhair M, Tam C, et al. Rituximab is associated with improved survival for aggressive B cell CNS lymphoma. Neuro Oncol 2013;15:1068-73.
- Holdhoff M, Ambady P, Abdelaziz A, Sarai G, Bonekamp D, Blakeley J, et al. High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology 2014;83:235-9.
- Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, et al. Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group experience. J Clin Oncol 2003;21:266-72.
- Lakshmaiah KC, Sathyanarayanan V, Babu KG, Lokanatha D, Lokesh KN, Babu MC, et al. Primary central nervous system lymphoma: An Indian perspective. Clin Cancer Invest J 2014;3:514.
- ;Paul T, Challa S, Tandon A, Panigrahi M, Purohit A. Primary central nervous system lymphomas: Indian experience, and review of literature. Indian J Cancer 2008;45:112-8.
- Pasricha S, Gupta A, Gawande J, Trivedi P, Patel D. Primary central nervous system lymphoma: A study of clinicopathological features and trend in Western India. Indian J Cancer 2011;48:199-203.
- Haldorsen IS, Aarseth JH, Hollender A, Larsen JL, Espeland A, Mella O. Incidence, clinical features, treatment and outcome of primary central nervous system lymphoma in Norway a ten-year national survey. Acta Oncol 2004;43:520-9.
- ;Agarwal PA, Menon S, Smruti BK, Singhal BS. Primary central nervous system lymphoma: A profile of 26 cases from Western India. Neurol India 2009;57:756-63.
- Gupta C, Shirazi N, Ahmad S, Burathoki SK, Verma SK. Primary CNS lymphoma in immuno-competent patients. J Indian Acad Clin Med 2011;12:193-5.
- ;Herrlinger U, Schabet M, Clemens M, Kortmann RD, Petersen D, Will BE, et al. Clinical presentation and therapeutic outcome in 26 patients with primary CNS lymphoma. Acta Neurol Scand 1998;97:257-64.
- ;ellinger K, Radaskiewicz T, Slowik F. Primary Malignant Lymphomas of the Central Nervous System in Man. In: Jellinger K, Seitelberger F. eds. Malignant Lymphomas of the Nervous System. Springer, Berlin, Heidelberg. Acta Neuropathologica 1975;6:Suppl 6:95-102.
- Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, et al. Primary central nervous system lymphoma: The Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 2006;24:5711-5.
- ;DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ, Radiation Therapy Oncology Group Study 93-10. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation therapy oncology group study 93-10. J Clin Oncol 2002;20:4643-8.
- Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol 1998;16:859-63.
- Yi JQ, Lin TY, He YJ, Huang HQ, Xia ZJ, Xia YF, et al. Primary central nervous system lymphoma – A report of 32 cases with literature review. Ai Zheng 2006;25:476-80.

| Figure 1:Kaplan–Meier estimates of progression-free survival for all patients (PFS – Progression-free survival)

| Figure 2:Kaplan–Mein curve comparing the survival between males and femalesKaplan–Meier estimates of overall survival for all patients (OS – Overall survival)

| Figure 3:Kaplan–Meier estimates of progression-free survival of patients based on International Extranodal Lymphoma Study Group risk scoring

| Figure 4:Kaplan–Meier estimates of overall survival of patients based on International Extranodal Lymphoma Study Group risk scoring
References
- Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985-1999. Neuro Oncol 2006;8:27-37.
- Shah GD, DeAngelis LM. Treatment of primary central nervous system lymphoma. Hematol Oncol Clin North Am 2005;19:611-27, v.
- Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG, et al. Descriptive epidemiology of primary brain and CNS tumors: Results from the central brain tumor registry of the United States, 1990-1994. Neuro Oncol 1999;1:14-25.
- Miller DC, Hochberg FH, Harris NL, Gruber ML, Louis DN, Cohen H, et al. Pathology with clinical correlations of primary central nervous system non-hodgkin's lymphoma. The Massachusetts general hospital experience 1958-1989. Cancer 1994;74:1383-97.
- Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg 1988;68:835-53.
- Krogh-Jensen M, D'Amore F, Jensen MK, Christensen BE, Thorling K, Pedersen M, et al. Clinicopathological features, survival and prognostic factors of primary central nervous system lymphomas: Trends in incidence of primary central nervous system lymphomas and primary malignant brain tumors in a well-defined geographical area. Population-based data from the Danish Lymphoma Registry, LYFO, and the Danish Cancer Registry. Leuk Lymphoma 1995;19:223-33.
- Fine HA, Mayer RJ. Primary central nervous system lymphoma. Ann Intern Med 1993;119:1093-104.
- Rubenstein JL, Treseler P, O'Brien JM. Pathology and genetics of primary central nervous system and intraocular lymphoma. Hematol Oncol Clin North Am 2005;19:705-17, vii.
- Ferreri AJ, Reni M, Villa E. Therapeutic management of primary central nervous system lymphoma: Lessons from prospective trials. Ann Oncol 2000;11:927-37.
- Ferreri AJ, Reni M, Pasini F, Calderoni A, Tirelli U, Pivnik A, et al. Amulticenter study of treatment of primary CNS lymphoma. Neurology 2002;58:1513-20.
- ;Joerger M, Huitema AD, Krähenbühl S, Schellens JH, Cerny T, Reni M, et al. Methotrexate area under the curve is an important outcome predictor in patients with primary CNS lymphoma: A pharmacokinetic-pharmacodynamic analysis from the IELSG no 20 trial. Br J Cancer 2010;102:673-7.
- Gregory G, Arumugaswamy A, Leung T, Chan KL, Abikhair M, Tam C, et al. Rituximab is associated with improved survival for aggressive B cell CNS lymphoma. Neuro Oncol 2013;15:1068-73.
- Holdhoff M, Ambady P, Abdelaziz A, Sarai G, Bonekamp D, Blakeley J, et al. High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology 2014;83:235-9.
- Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, et al. Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group experience. J Clin Oncol 2003;21:266-72.
- Lakshmaiah KC, Sathyanarayanan V, Babu KG, Lokanatha D, Lokesh KN, Babu MC, et al. Primary central nervous system lymphoma: An Indian perspective. Clin Cancer Invest J 2014;3:514.
- ;Paul T, Challa S, Tandon A, Panigrahi M, Purohit A. Primary central nervous system lymphomas: Indian experience, and review of literature. Indian J Cancer 2008;45:112-8.
- Pasricha S, Gupta A, Gawande J, Trivedi P, Patel D. Primary central nervous system lymphoma: A study of clinicopathological features and trend in Western India. Indian J Cancer 2011;48:199-203.
- Haldorsen IS, Aarseth JH, Hollender A, Larsen JL, Espeland A, Mella O. Incidence, clinical features, treatment and outcome of primary central nervous system lymphoma in Norway a ten-year national survey. Acta Oncol 2004;43:520-9.
- ;Agarwal PA, Menon S, Smruti BK, Singhal BS. Primary central nervous system lymphoma: A profile of 26 cases from Western India. Neurol India 2009;57:756-63.
- Gupta C, Shirazi N, Ahmad S, Burathoki SK, Verma SK. Primary CNS lymphoma in immuno-competent patients. J Indian Acad Clin Med 2011;12:193-5.
- ;Herrlinger U, Schabet M, Clemens M, Kortmann RD, Petersen D, Will BE, et al. Clinical presentation and therapeutic outcome in 26 patients with primary CNS lymphoma. Acta Neurol Scand 1998;97:257-64.
- ;ellinger K, Radaskiewicz T, Slowik F. Primary Malignant Lymphomas of the Central Nervous System in Man. In: Jellinger K, Seitelberger F. eds. Malignant Lymphomas of the Nervous System. Springer, Berlin, Heidelberg. Acta Neuropathologica 1975;6:Suppl 6:95-102.
- Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, et al. Primary central nervous system lymphoma: The Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 2006;24:5711-5.
- ;DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ, Radiation Therapy Oncology Group Study 93-10. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation therapy oncology group study 93-10. J Clin Oncol 2002;20:4643-8.
- Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol 1998;16:859-63.
- Yi JQ, Lin TY, He YJ, Huang HQ, Xia ZJ, Xia YF, et al. Primary central nervous system lymphoma – A report of 32 cases with literature review. Ai Zheng 2006;25:476-80.


 PDF
PDF  Views
Views  Share
Share

