Clinicopathological features and outcomes in advanced nonsmall cell lung cancer with tailored therapy
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(04): 242-250
DOI: DOI: 10.4103/0971-5851.195735
Abstract
Context: Lung cancer is an important cause of cancer-related deaths worldwide. There is an increasing incidence of lung cancer in never smokers and a shift of histology from squamous cell to adenocarcinoma globally in the recent past. Data on treatment outcomes with newer platinum doublets is scant from India. Aims: To study the clinicopathological features, response rates (RRs), progression-free survival (PFS), overall survival (OS), and the 1, 2, and 3 years survival, in patients with advanced nonsmall cell lung cancer (NSCLC). Materials and Methods: Data of all patients who received chemotherapy for Stage IIIB and IV NSCLC between January 2010 and June 2014 were retrospectively analyzed. Statistical Analysis Used: Univariate analysis for OS was done by plotting Kaplan–Meier curves and the log-rank test was used to calculate P values. Logistic regression analysis for OS was carried out using MedCalc statistical software. Results: A total of 353 patients received chemotherapy. Of these, 256 were evaluable for outcome parameters. The median age at presentation was 58 years with a male:female ratio of 2.53:1. The smoker:nonsmoker ratio was 1:1. Adenocarcinomatous histology was the most common both in smokers and nonsmokers reported in 70.8% patients. Epidermal growth factor receptor (EGFR) mutation and echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase translocation were seen in 35% and 3% of patients, respectively. The RR, median PFS, OS, 1, 2, and 3 years survival were 80%, 8 months, 12.1 months, 51.5%, 12.7%, and 4.2%, respectively. There was no significant survival difference among the treatment regimen used but the response to I line chemotherapy impacted survival. Female gender, performance status, and nonsquamous histology were significant predictors of OS (P = 0.0443, P = 0.0003, P = 0.048, respectively). Conclusions: There was an increase in the incidence of nonsmokers. Adenocarcinoma was the most common histology in both smokers and nonsmokers. Treatment outcomes in advanced lung cancer were better compared to the past with the advent of newer platinum doublets and EGFR tyrosine kinase inhibitors. The response to first-line chemotherapy significantly impacts outcomes in advanced NSCLC.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
Lung cancer is an important cause of cancer-related deaths worldwide. There is an increasing incidence of lung cancer in never smokers and a shift of histology from squamous cell to adenocarcinoma globally in the recent past. Data on treatment outcomes with newer platinum doublets is scant from India.
Aims:
To study the clinicopathological features, response rates (RRs), progression-free survival (PFS), overall survival (OS), and the 1, 2, and 3 years survival, in patients with advanced nonsmall cell lung cancer (NSCLC).
Materials and Methods:
Data of all patients who received chemotherapy for Stage IIIB and IV NSCLC between January 2010 and June 2014 were retrospectively analyzed.
Statistical Analysis Used:
Univariate analysis for OS was done by plotting Kaplan–Meier curves and the log-rank test was used to calculate P values. Logistic regression analysis for OS was carried out using MedCalc statistical software.
Results:
A total of 353 patients received chemotherapy. Of these, 256 were evaluable for outcome parameters. The median age at presentation was 58 years with a male:female ratio of 2.53:1. The smoker:nonsmoker ratio was 1:1. Adenocarcinomatous histology was the most common both in smokers and nonsmokers reported in 70.8% patients. Epidermal growth factor receptor (EGFR) mutation and echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase translocation were seen in 35% and 3% of patients, respectively. The RR, median PFS, OS, 1, 2, and 3 years survival were 80%, 8 months, 12.1 months, 51.5%, 12.7%, and 4.2%, respectively. There was no significant survival difference among the treatment regimen used but the response to I line chemotherapy impacted survival. Female gender, performance status, and nonsquamous histology were significant predictors of OS (P = 0.0443, P = 0.0003, P = 0.048, respectively).
Conclusions:
There was an increase in the incidence of nonsmokers. Adenocarcinoma was the most common histology in both smokers and nonsmokers. Treatment outcomes in advanced lung cancer were better compared to the past with the advent of newer platinum doublets and EGFR tyrosine kinase inhibitors. The response to first-line chemotherapy significantly impacts outcomes in advanced NSCLC.
INTRODUCTION
Lung cancer is the most common cause of cancer death worldwide of which nonsmall cell lung cancer (NSCLC) predominates.[1] While there has been a substantial decline in lung cancer rates in developed countries,[2] incidence rates are reportedly rising in newly industrialized and developing countries such as China and India.[3] Approximately, 70,000 new cases of lung cancer were diagnosed in India in 2012.[4] There is increasing the incidence of lung cancer in never smokers[5,6] and also a shift of histology from squamous cell carcinoma to adenocarcinoma.[7] The overall ratio of mortality to incidence is high because of presentation in an advanced stage.[8]
Epidermal growth factor receptor (EGFR) mutation and echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) translocation are the two main oncogenic drivers identified in the pathogenesis of NSCLC for which targeted therapy is available.[9,10] With the advent of EGFR tyrosine kinase inhibitors (TKIs), pemetrexed- and taxane-based platinum doublet, survivals in NSCLC were significantly improved along with marked improvement in quality of life.[11,12,13] Survival and safety data using these newer platinum doublets and targeted therapy are scant in India.
Usage of EGFR TKI in mutation-positive advanced NSCLC patients had resulted in dramatic improvements in response rates (RRs) and survivals, and they had also become a reasonable option in poor performance status who are unfit for chemotherapy.[14,15]
The primary objectives of this analysis were to study the RRs, median progression-free survival (PFS), and overall survival (OS) in advanced NSCLC patients treated with platinum-based chemotherapy and targeted therapy with EGFR TKI and ALK inhibitors.
The secondary objectives were to study the demographic, clinicopathological features, toxicity profile and predictors of survival with the newer platinum doublets, and EGFR TKIs.
MATERIALS AND METHODS
Data from medical records of patients with advanced NSCLC who received chemotherapy between January 2010 and June 2014 were retrieved, and the Institutional Ethical Committee approved the study.
All patients with the diagnosis of advanced NSCLC were analyzed for demographic and clinicopathological features and those patients who had taken at least two cycles of chemotherapy or 2 months of EGFR TKI and had a contrast-enhanced computed tomography (CECT) scan for response evaluation were eligible for the assessment of outcome parameters, namely, RR, PFS, OS, as well as survival rates at 1, 2, and 3 years. Data of patients who did not receive at least two cycles or a response evaluation were censored for outcome parameters.
The diagnosis of NSCLC was confirmed either by fine-needle aspiration or biopsy of lung mass or pleural fluid cytology or cell block analysis. Patients with indeterminate cytology or histology and poorly differentiated histology were diagnosed as NSCLC-not otherwise specified (NSCLC-NOS). The staging investigations included CECT scan of the chest and upper abdomen and bone scan or positron emission tomography-computed tomography (CT) scan. CT scan or magnetic resonance imaging scan of the brain was done whenever appropriate. Other investigations included complete blood counts, liver, and renal functions tests. EGFR mutation analysis was done using a real-time polymerase chain reaction. ALK screening was done through immunohistochemistry (IHC) and positive cases were confirmed by fluorescent in situ hybridization. Staging was done according to AJCC 7th Edition of lung cancer staging.[16]
Informed consent was taken from all patients before administration of chemotherapy patients were treated with various regimens administered intravenously or orally. Platinum doublets used were cisplatinum 75 mg/m2 D1/carboplatin (AUC 5) D1 + pemetrexed 500 mg/m2 D1[11]/paclitaxel 175 mg/m2 D1[12]/albumin-bound paclitaxel 260 mg/m2 D1[17]/gemcitabine 1 g/m2 D1 and D8.[11] EGFR TKIs and ALK inhibitors used were gefitinib 250 mg or erlotinib 150 mg once daily and crizotinib 250 mg once daily. Vitamin B12 and folate supplementation were given before and during pemetrexed-based chemotherapy and antihistamines, and steroids were given prophylactically before paclitaxel administration. Chemotherapy dosages were modified in patients with renal and liver dysfunction.
Patients were also given radiotherapy (RT) wherever it was indicated, with palliative intent, for primary or metastatic sites. Patients with anemia received transfusions, febrile neutropenia received growth factor support with antibiotics. Pleural fluid drainage was done in patients with symptomatic pleural effusion. Patients were given a maximum of 4–6 cycles of chemotherapy followed by continuous or switch maintenance until progression, based on the response evaluation and EGFR mutation status.
Response evaluation was performed after every 2–3 cycles of chemotherapy by a clinical examination and CECT of the chest and upper abdomen.
The following response criteria were used
Revised RECIST guideline version 1.1 was used to define response evaluation criteria.[12]
A complete response (CR) was defined as disappearance of all the lesions on radiology. Partial response (PR) was defined as a decrease of 30% in the sum of the longest diameter of all target lesions. Progressive disease (PD) was defined as an increase of 20% in the sum of the longest diameters of the target lesions or appearance of a new lesion at any time during or after therapy. Stable disease (SD) was defined as patients who did not fit into either PR or PD.
PFS was defined as the time from start of chemotherapy to the time that PD was documented, death, or lost to follow-up. OS was defined as the time from start of chemotherapy to death due to any cause or lost to follow-up.
Statistical methods
GraphPad Software Quick Cals online calculator was used to calculate the P values for the categorical and continuous variables. For continuous variables, the P value was calculated using the unpaired t-test to compare the means. For categorical data such as stage, smoking, sex, performance status, and RRs, the two-tailed P value was calculated using Fisher's exact test and 2 × 2 contingency table.
Univariate and multivariate analysis were done to assess the effect of age, sex, smoking status, performance status, stage, and chemotherapy regimens on OS. Patients were also compared for all outcome parameters with respect to whether they were treated with a platinum-based doublet or EGFR TKI.
GraphPad Prism software for Windows Version 6 was used to plot the Kaplan–Meier curves for PFS and OS (GraphPad Software, La Jolla California USA, www.graphpad.com). Univariate analysis for OS was done by plotting Kaplan–Meier curves, and the log-rank test was used to calculate P values. Logistic regression analysis for OS was carried out using MedCalc demo version statistical software 16.4.3 using the same independent variables after coding (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2016). P < 0.05 was considered as statistically significant.
RESULTS
Patient characteristics
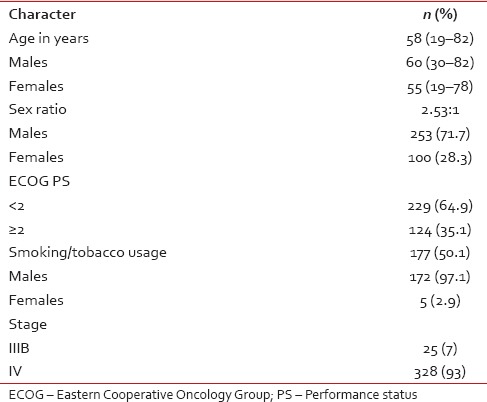
Three hundred and fifty-three patients who received chemotherapy for advanced NSCLC between January 2010 and August 2014 were retrospectively analyzed. The flow diagram was shown in Figure 1. The median age of patients was 58 years (range, 19–82) with a male: female ratio of 2.53:1. The baseline characteristics of all patients are in Table 1.
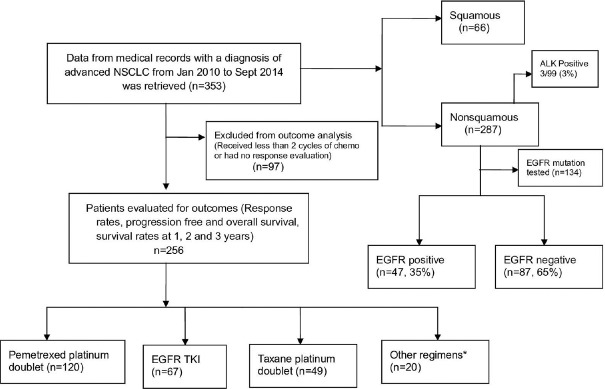
| Fig. 1 Flow diagram of study patients. Other regimens* – gemcitabine-platinum doublet, carboplatin and etoposide, cisplatin, and etoposide. NSCLC – Nonsmall cell lung cancer; EGFR – Epidermal growth factor receptor; TKI – Tyrosine kinase inhibitor; ALK – Anaplastic lymphoma kinase
Table 1
Demographic characteristics of all patients (n=353)
Of this, 256 patients took at least 2 cycles of chemotherapy and had radiological response evaluation. These patients were eligible for the evaluation of outcome parameters such as RR, median PFS, OS, 1 year, 2 years, and 3 years survival. 97 patients who had received < 2 cycles or were lost to follow-up without having a radiological response evaluation were not eligible for outcome parameters.
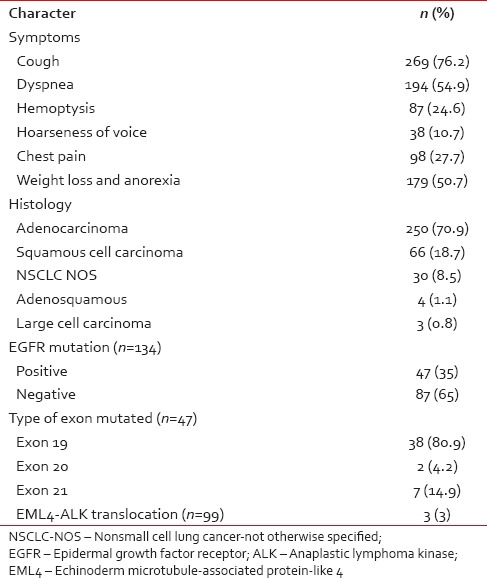
Cough (76.2%) was the most common symptom at presentation followed by dyspnea, weight loss, anorexia, and chest pain. Hemoptysis and hoarseness of voice are present in 24.6% and 10.7% patients, respectively. Central nervous system symptoms at presentation were seen in 20 (5.6%) patients, of which headache was the most common.
Forty-three (12.1%) patients were started on antituberculosis treatment outside before diagnosis of NSCLC at our institute. The median duration of delay in diagnosis is 3 months (1–12 months). Smoker: nonsmoker ratio in the present study was 1:1 with smoking history seen in 168 (66%) males and 9 (9%) females, respectively. The median age at presentation in smokers and nonsmokers was 60 years (35–82) and 55 years (19–78), respectively.
The diagnosis of NSCLC was based on biopsy in 200 (56.7%) and cytology in 153 (43.3%) patients. The most common histological diagnosis was adenocarcinoma 250 (70.8%) followed by squamous cell carcinoma 66 (18.8%), NSCLC-NOS (8.5%), adenosquamous (1.1%), and large cell carcinoma (0.8%). IHC was done in 212/353 (60%) patients and TTF-1, napsyn, and p63 were positive, respectively, in 68% (148/212), 50% (32/64), and 27% (39/143) tested for immunohistochemical markers. Out of the 266 adenocarcinoma and 50 squamous cell carcinoma patients, smoking history is seen in 47.4% and 70%, respectively. The median duration of pack-years of smoking was 30 pack years. Clinicopathological features were tabulated in Table 2.
Table 2
Clinicopathologic features (n=353)
The comorbidities of the patients were tabulated in Table 3. Opposite lung, bone, pleural effusion, adrenal gland, liver, and brain metastases were seen in 126 (38.6%), 109 (33.4%), 107 (32.8%), 57 (17.4%), 35 (10.7%), and 33 (10.1%) patients, respectively.
Table 3
Comorbid conditions of all patients (n=353)

Epidermal growth factor receptor mutation and echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase translocation analysis
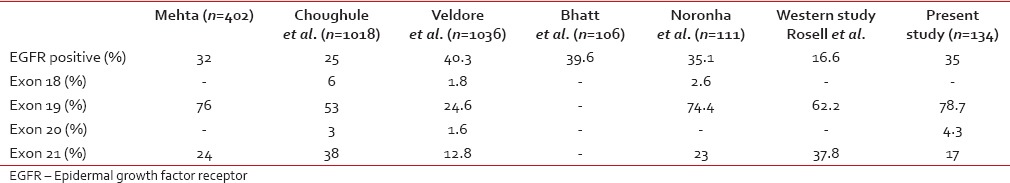
EGFR mutation analysis was done in 134 of 303 nonsquamous patients. Of the 134 patients, 46 (34.3%) were EGFR mutation positive. The most common exon mutated was 19 (78.7%. EML4-ALK translocation was seen in 3 (3%) out of the 99 patients tested.
Treatment results for all patients
One hundred and thirty-four patients had at least a CR (3/256) or PR (131/256), with an overall RR of 52.3% (134/256). SD and PD were seen in 71/256 (27.7%) and 51/256 (20%) patients, respectively. The median PFS was 8 months (range 2–58) and OS was 12.1 months (range 2–70). The median PFS and OS were shown in Figure 2 and Figure 3 respectively. The 1 year, 2 years, and 3 years survival was 51.5%, 12.9%, and 4.2%, respectively. Treatment outcomes for all patients were tabulated in Table 4.
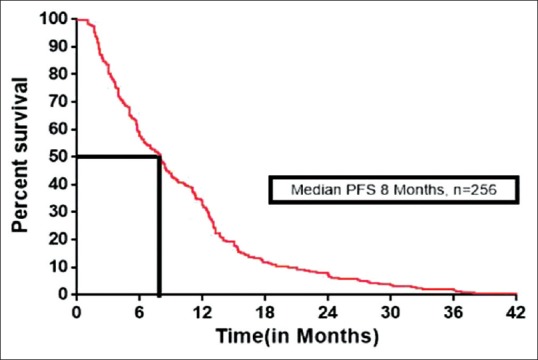
| Fig. 2 Kaplan–Meier estimates of progression-free survival for all patients. PFS – Progression-free survival
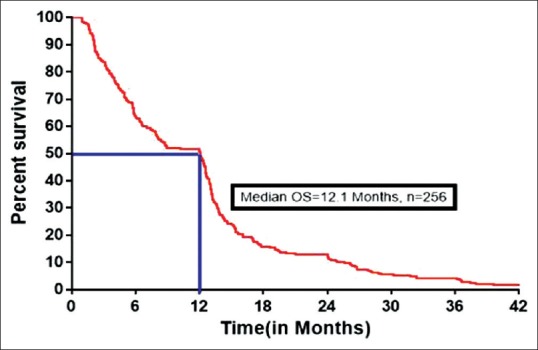
| Fig. 3 Kaplan–Meier estimates of overall survival for all patients. OS – Overall survival
Table 4
Treatment outcomes for all patients (n=256)

Out of 256 patients who were evaluated for outcome parameters, 205 (80%) patients who had PR or SD on response evaluation were eligible for maintenance therapy. Of the 205 patients, 105 (51.2%) patients received continuation or switch maintenance. Fifty-eight (22.6%) patients were able to receive second-line chemotherapy at disease progression. The median number of cycles of initial chemotherapy was 4 cycles followed which maintenance was started based on EFGR mutation status and the median duration of follow-up was 11.2 months (range, 2–70 months).
The overall RR, median PFS, and OS in EGFR mutation positive patients treated with EGFR TKI were 82%, 13.5 months (range, 2.8–36), and 16.8 months (range, 4.8–40), respectively.
Univariate and multivariate analysis of variables for overall survival
Univariate analysis was performed for age (< 60 years vs. ≥60 years), gender (male vs. female), smoking status (yes vs. no), Stage (IIIB vs. IV), performance status ( < 2 vs. ≥2), histology (nonsquamous vs. squamous), treatment regimens used for OSs.
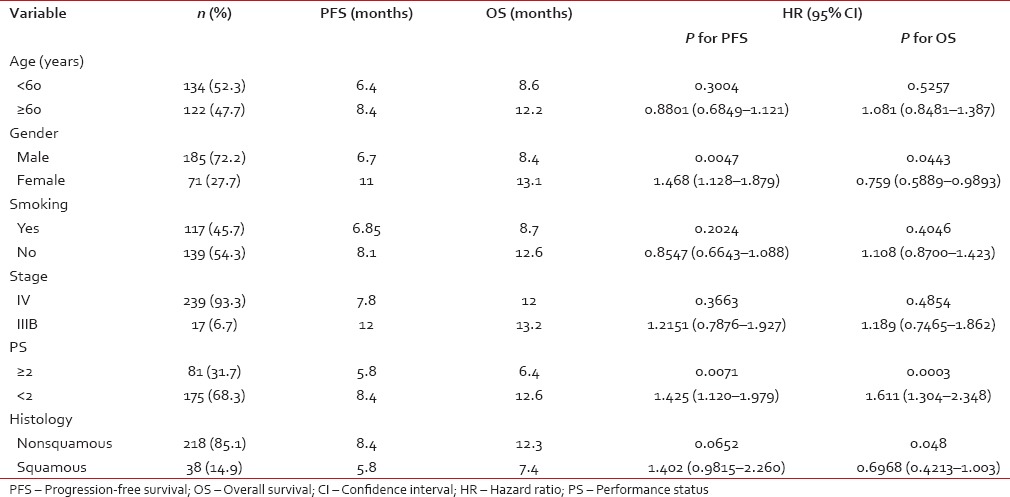
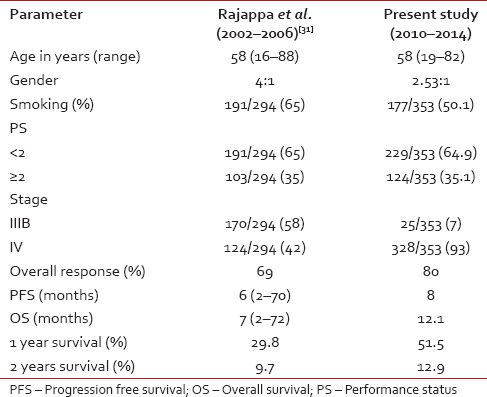
On univariate analysis, the strongest predictors for OS were female gender, performance status, and nonsquamous histology (P = 0.04, P = 0.0003, and P = 0.048 respectively). Age, stage, smoking status, and treatment regimens did not predict significantly for OS (P = 0.5257, P = 0.4854, P = 0.404, and P = 0.1502, respectively). On multivariate analysis also, female gender, performance status, and nonsquamous histology were significant for OS (P = 0.0415, P = 0.0018, and P = 0.0231, respectively). Comparison of present study with our previous data was tabulated in Table 5.
Table 5
Univariate analysis of treatment variables (n=256)
Effect of chemotherapy regimen and treatment response on overall survival
The outcomes for patients treated with various doublets were analyzed. The median OS for patients treated with pemetrexed-based platinum, taxane-based platinum, and EFGR TKI were 12.3, 7.4, and 12.5, respectively which was not significant statistically (P = 0.1502). OS based on chemotherapy regimen was shown in Figure 4.
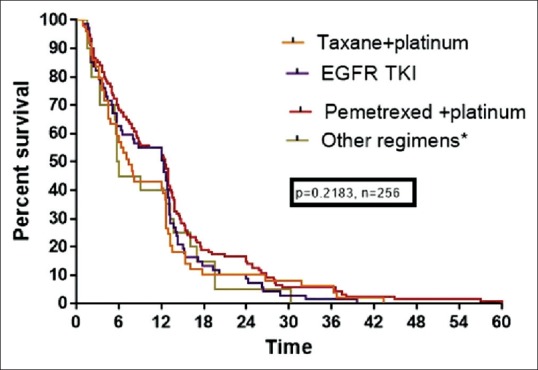
| Fig. 4 Kaplan–Meier estimates of survival (overall survival) of platinum doublets and Epidermal growth factor receptor tyrosine kinase inhibitor. EGFR – Epidermal growth factor receptor; TKI – Tyrosine kinase inhibitor
The median OS for patients who had CR/PR, SD, and PD were 13.1, 8, and 4.1 months, respectively which was statistically significant (P ≤ 0.0001). OS based on response to 1st line is shown in Figure 5.
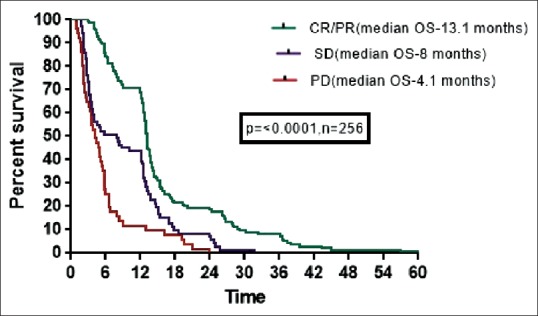
| Fig. 5 Kaplan–Meier estimates of survival (overall survival) of all patients based on response to first-line treatment. CR – Complete response; PR – Partial response; SD – Stable disease; PD – Progressive disease
Radiotherapy
Out of 256 patients, 54 (21%) patients received RT. Forty-nine (19.1%) patients received RT for extrathoracic disease (brain and bone) and 5 (1.9%) patients received RT for locoregional disease.
Adverse events following chemotherapy and tyrosine kinase inhibitors
The most common adverse events were fatigue (10%), vomiting (9%). Grade 2–3 neuropathy and alopecia is seen in 12.2% and 8.1% with taxane-based treatment. Myelosuppression is seen in 8.1% and 4% patients treated with taxane and pemetrexed-based platinum treatment, respectively. Two patients had megaloblastic anemia. Skin rash is the most common adverse effect with EGFR TKI, seen in 32% of patients of which 4.5% needed dose reduction or stoppage of drug.
DISCUSSION
This is a retrospective analysis of patients with advanced lung cancer. Typically, palliation of symptoms and most objective responses in lung cancer are reported to occur during the first 2 cycles of chemotherapy. Hence, in our study, we had evaluated all patients who received at least 2 cycles of chemotherapy and had an objective response evaluation for outcome parameters.
Table 6
Comparision with other Indian studies
 EGFR mutational analysis was done in only 50% of the patients with nonsquamous histology. The nonavailability of mutation analysis in the 50% patients is due to use of cytology as the diagnostic modality in some cases and inadequate biopsy tissue. Among those tested, 35% (47/134) of patients were EGFR mutation positive, which is comparable to reports by Bhatt et al., Veldore et al., Mehta and Choughule et al., who reported the frequency of EGFR mutation between 25% and 40%,[26,27,28,29] respectively. Exon 19 was the most common mutation seen in 78% of patients. EGFR mutation status comparison with other studies was tabulated in Table 7. The presence of EML4-ALK translocation in 3% (3/99) of patients tested for translocation is similar to the reports by Kwak et al.[10] and Doval et al.[30]
EGFR mutational analysis was done in only 50% of the patients with nonsquamous histology. The nonavailability of mutation analysis in the 50% patients is due to use of cytology as the diagnostic modality in some cases and inadequate biopsy tissue. Among those tested, 35% (47/134) of patients were EGFR mutation positive, which is comparable to reports by Bhatt et al., Veldore et al., Mehta and Choughule et al., who reported the frequency of EGFR mutation between 25% and 40%,[26,27,28,29] respectively. Exon 19 was the most common mutation seen in 78% of patients. EGFR mutation status comparison with other studies was tabulated in Table 7. The presence of EML4-ALK translocation in 3% (3/99) of patients tested for translocation is similar to the reports by Kwak et al.[10] and Doval et al.[30]Table 7
Comparision of epidermal growth factor receptor mutation status in present study with other studies
 The overall RR (complete and PR) of 52.3% observed in the present study is significantly higher compared to a previous study from this center by Rajappa et al.[31] This is because of use of newer platinum doublets and EGFR TKI in most of the patients. The survival rates at 1, 2, and 3 years were also markedly improved with the use of these chemotherapeutic agents. Among the various regimens used, treatment with pemetrexed-based platinum doublet and EGFR TKI had higher PFS and OS, compared to treatment with taxane- and gemcitabine-based doublet (OS: 12.3, 12.5, 7.9 and 5.9 months, respectively, for pemetrexed, EGFR TKI, taxane, and gemcitabine, P = 0.2183). Although this was statistically insignificant, Grade 2–3 neuropathy, myelosuppression, and alopecia were higher in patients treated with taxane-based platinum doublet, compared to pemetrexed and EGFR TKI, suggesting pemetrexed and EGFR TKI were better tolerated and less toxic regimens with significant benefits on quality of life.
The overall RR (complete and PR) of 52.3% observed in the present study is significantly higher compared to a previous study from this center by Rajappa et al.[31] This is because of use of newer platinum doublets and EGFR TKI in most of the patients. The survival rates at 1, 2, and 3 years were also markedly improved with the use of these chemotherapeutic agents. Among the various regimens used, treatment with pemetrexed-based platinum doublet and EGFR TKI had higher PFS and OS, compared to treatment with taxane- and gemcitabine-based doublet (OS: 12.3, 12.5, 7.9 and 5.9 months, respectively, for pemetrexed, EGFR TKI, taxane, and gemcitabine, P = 0.2183). Although this was statistically insignificant, Grade 2–3 neuropathy, myelosuppression, and alopecia were higher in patients treated with taxane-based platinum doublet, compared to pemetrexed and EGFR TKI, suggesting pemetrexed and EGFR TKI were better tolerated and less toxic regimens with significant benefits on quality of life.iPASS[12] and iTARGET[13] trials reported an overall RR of 52% and 65%–70%, respectively in EGFR mutation positive patients treated with gefitinib. The ORR of 82% in EGFR mutation positive patients treated with gefitinib or erlotinib was higher in this analysis compared to these reports, but the OS seen in our study was slightly lower compared to iPASS trial.[12]
Univariate and multivariate analysis for various predictors of survival showed that female gender, performance status at presentation, and nonsquamous histology predicted for OS significantly. The possible reason for better survival in females and nonsquamous histology would be due to more number of mutation positive patients and use of targeted therapy in these patients. The response to first-line chemotherapy also had a significant impact on the survival with patients having complete or PR had better OS than SD and PD. This was similar to a meta-analysis by Johnson et al.[32] and a study by Sirohi et al.,[33] who reported that patients with PR after 2 cycles have a better response and longer survival compared to patients with SD.
Compared to the previous data from this center presented by Rajappa et al. in 2008,[31] there was an increase in incidence of women and never-smokers and also a marked improvement in PFS, OS and survival rates at 1, 2, and 3 years, showing the effectiveness of newer platinum doublets compared to I generation platinum doublet. Comparision with previous data by Rajappa et al was shown in Table 8. Only 58 patients received second-line chemotherapy at progression, and the probable reasons were deterioration of PS or lack of adequate finances.Table 8
Comparision of present study with our previous data
The drawbacks of the present study are that it is retrospective, with limited data on adverse events of chemotherapy and lack of information on quality of life. There is underreporting of both hematologic and nonhematologic adverse events because of inadequate documentation in the case files.
CONCLUSIONS
The findings of our study have significant implications for clinical practice. There was an increase in the incidence of lung cancer in never smokers. Adenocarcinoma was the most common histology of lung cancer. The survival in lung cancer is significantly increased with the use of newer platinum doublets and targeted therapy compared to the past. Female gender and good performance status were strong predictors for better survivals in advanced NSCLC. Pemetrexed and EGFR TKI were well-tolerated agents with less adverse events.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.

| Fig. 1 Flow diagram of study patients. Other regimens* – gemcitabine-platinum doublet, carboplatin and etoposide, cisplatin, and etoposide. NSCLC – Nonsmall cell lung cancer; EGFR – Epidermal growth factor receptor; TKI – Tyrosine kinase inhibitor; ALK – Anaplastic lymphoma kinase

| Fig. 2 Kaplan–Meier estimates of progression-free survival for all patients. PFS – Progression-free survival

| Fig. 3 Kaplan–Meier estimates of overall survival for all patients. OS – Overall survival

| Fig. 4 Kaplan–Meier estimates of survival (overall survival) of platinum doublets and Epidermal growth factor receptor tyrosine kinase inhibitor. EGFR – Epidermal growth factor receptor; TKI – Tyrosine kinase inhibitor

| Fig. 5 Kaplan–Meier estimates of survival (overall survival) of all patients based on response to first-line treatment. CR – Complete response; PR – Partial response; SD – Stable disease; PD – Progressive disease


 PDF
PDF  Views
Views  Share
Share

