Clinico radiological and Pathological Characteristics of Inflammatory Myofibroblastic Tumors in Children: A Retrospective Study
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 261-265
DOI: DOI: 10.4103/ijmpo.ijmpo_178_16
Abstract
Purpose: Inflammatory myofibroblastic tumors (IMTs) are rare, benign lesions most often seen in the lung of young adults but can occur in children, in various sites. They mimic, clinically and radiologically, malignant tumors – especially sarcomas and lymphomas. The aim was to review the clinical, radiological, and pathological data of children with a diagnosis of IMT referred to our department. Materials and Methods: This retrospective study was conducted at the Department of Medical and Paediatric Oncology, Regional Cancer Centre, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India from January 2014 to December 2015. Results: Among 288 pediatric tumors registered during the study, 5 (1.73%) had the diagnosis of IMTs. The main symptoms were abdominal distension and pain in 60% (three cases), breathlessness and cough in 20% (one case), and right axillary area swelling in 20% (one case). In three patients, complete surgical excision was done, whereas another patient with retroperitoneal mass had the residual disease and received chemotherapy followed by complete second surgery. In the case of mediastinal IMT, surgery was followed by local radiotherapy. At present, four patients are disease-free and one patient with mediastinal IMT has the residual progressive disease. Conclusion: On presentation, IMT can constitute a formidable challenge, from diagnosis through to treatment.
Keywords
Inflammatory myofibroblastic tumors - macrolides - pseudotumors - steroids - tyrosine kinasePublication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Purpose:
Inflammatory myofibroblastic tumors (IMTs) are rare, benign lesions most often seen in the lung of young adults but can occur in children, in various sites. They mimic, clinically and radiologically, malignant tumors – especially sarcomas and lymphomas. The aim was to review the clinical, radiological, and pathological data of children with a diagnosis of IMT referred to our department.
Materials and Methods:
This retrospective study was conducted at the Department of Medical and Paediatric Oncology, Regional Cancer Centre, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India from January 2014 to December 2015.
Results:
Among 288 pediatric tumors registered during the study, 5 (1.73%) had the diagnosis of IMTs. The main symptoms were abdominal distension and pain in 60% (three cases), breathlessness and cough in 20% (one case), and right axillary area swelling in 20% (one case). In three patients, complete surgical excision was done, whereas another patient with retroperitoneal mass had the residual disease and received chemotherapy followed by complete second surgery. In the case of mediastinal IMT, surgery was followed by local radiotherapy. At present, four patients are disease-free and one patient with mediastinal IMT has the residual progressive disease.
Conclusion:
On presentation, IMT can constitute a formidable challenge, from diagnosis through to treatment.
Introduction
Inflammatory myofibroblastic tumor (IMT) is a rare clinical entity that encompasses a wide spectrum of myofibroblastic proliferation along with a varied amount of inflammatory infiltrate. First described by Brunn et al. in 1939 as a primary lung tumor, IMT can occur in nearly any body organ.[1] Previously called by many names such as inflammatory pseudotumor, fibrous xanthoma, pseudosarcoma, lymphoid hamartoma, and inflammatory myofibrohistiocytic proliferation the diverse nomenclature is mostly descriptive and reflects the uncertainty regarding true biologic nature of these lesions.[2] The term “inflammatory pseudotumor” was coined by Umiker et al. in 1954 who described four inflammatory tumors of the lung simulating xanthoma, fibroma, or plasma cell tumor. IMTs predominantly occur in children and young adults, but any age group can be affected.[3] IMTs occur more commonly in women. Although considered as benign tumors, IMTs have a propensity toward recurrence and persistent local growth.
The pathologically IMT is composed of cytologically bland spindled myofibroblasts with admixed inflammatory cells.[4] The spindle cells contain large vesicular oval nuclei with no hyperchromasia and small nucleoli. Variable amounts of pale eosinophilic cytoplasm and variable number of mitotic figures may be seen. Inflammatory cells are predominantly lymphocytes and plasma cells but may include neutrophils and eosinophils also. These cells may form germinal centers. Foamy histiocytes are occasionally present.[5] The patterns of cell mixtures may either take the form of loose or myxoid stroma with prominent vascularity or compact spindle cells or densely collagenous with fewer spindle cells and inflammatory cells.[6,7] Immunohistochemistry reveals reactivity of IMTs to actin in the majority of the cases and to vimentin in all cases reflecting the myofibroblastic nature of the cells.[8,9,10] Cytokeratin and desmin may be reactive in some cases.
Aims and objectives
- To describe the prevalence and clinical, radiological, and pathological characteristics of inflammatory fibroblastic tumors in children.
Materials and Methods
This study was carried out in the Department of Medical Oncology Sher-I-Kashmir Institute of Medical Sciences, Srinagar, India, which is one of the astute tertiary care centers in North India with a dedicated Regional Cancer Centre (RCC) catering to the needs of more than 13 million people. In this study, all the pediatric cancer patients registered with RCC from January 2014 to December 2015 were included in the study. All the clinical signs and symptoms and relevant diagnostic methods and their results of patients with a diagnosis of the inflammatory fibroblastic tumor were documented, and their treatment details noted.
Results
Among 288 pediatric tumors registered during the study, 5 (1.73%) had the diagnosis of IMTs. There were three male and two female children (M:f ratio 1.5:1). The mean age was 5.32 years (range: 2–9 years). The main symptoms were abdominal distension and pain in 60% (three cases), breathlessness and cough in 20% (one case), and right axillary area swelling in 20% (one case). On computed tomography of chest and abdomen, one patient had mediastinal widening with the impression of lymphoma, two patients were labeled as having retroperitoneal sarcoma, and one patient had an ileal growth with the impression of Burkitt lymphoma [Figures [Figures11–3].
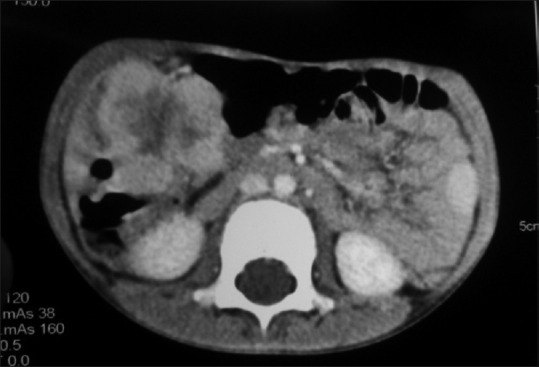
| Figure 1:Contrast-enhanced computed tomography of abdomen and pelvis revealed a 7 cm × 5 cm × 5 cm lobulated hyper enhancing mass lesion with central necrosis arising from bowel wall extraluminally in ileocecal area suggestive of gut lymphoma

| Figure 2:Low power view of Histopathological examination (HPE) showing spindle cells with inflammatory background of plasma cells, lymphocytes, and neutrophils
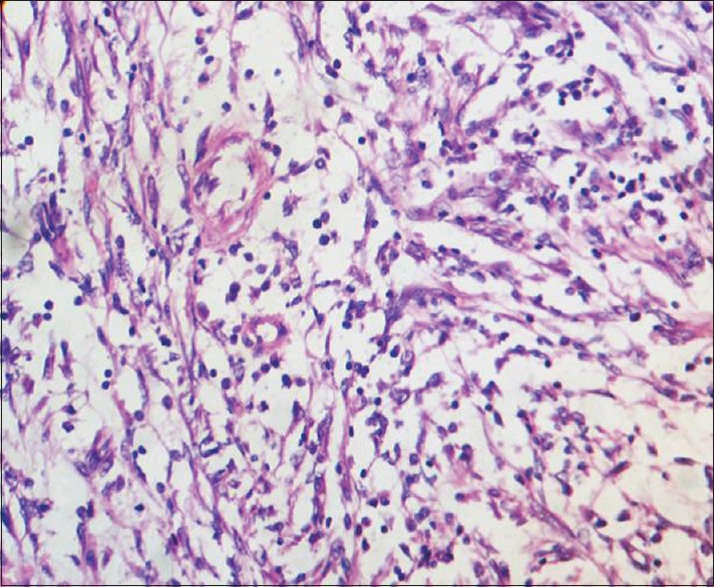
| Figure 3:High power view of HPE showing spindle cells with inflammatory background of plasma cells lymphocytes and neutrophils
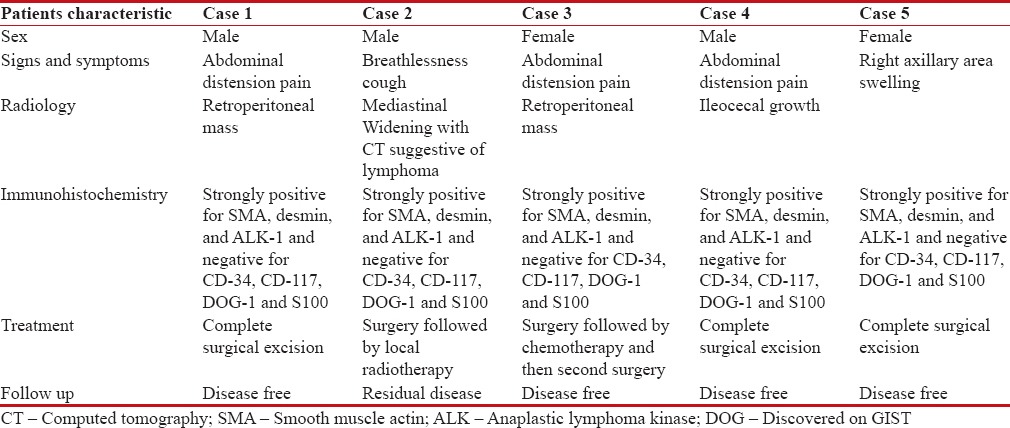
In one patient with right axillary swelling, lymphoma was suspected. In only one patient (20%), initial histopathology was reported as IMT (retroperitoneal lesion), whereas in rest of the cases, it was only after review with immunohistochemistry from an oncopathologist that diagnosis of IMT was made. On immunohistochemistry, in all patients, tumor cells were strongly positive for smooth muscle actin, desmin, and anaplastic lymphoma kinase (ALK)-1 and negative for CD-34, CD-117, discovered on GIST and S100. In three patients, complete surgical excision was done (one with axillary mass and two with abdominal disease). One patient with retroperitoneal mass had residual disease and received chemotherapy followed by complete second surgery. In the case of mediastinal IMT, surgery was followed by local radiotherapy. At present, four patients are disease-free and one patient with mediastinal IMT has residual progressive disease. The summary of this study is given in Table 1.
Table 1
Clinico-radiological characteristics of inflammatory myofibroblastic tumor patients in our study
|
Discussion
IMT is a rare tumor of childhood and adolescence. Although they occur predominantly in lung, they may occur in any organ. Extrapulmonary IMT occurs commonly in the abdomen or in soft tissues but may occur anywhere. Surprisingly, in our study, there was no pulmonary IMT; instead, there was one mediastinal IMT, two retroperitoneal, one axillary, and one ileocecal. This may be due to overall low prevalence of IMTs. These lesions are great mimickers and may escape diagnosis.[11] Oeconomopoulou et al. reported a young child who was initially diagnosed to have acute appendicitis but on laparotomy was found to have a tumor that was histology and immunohistochemistry was found to be IMT. Similarly, in our case, none of the patients was initially suspected to have IMT. In fact, ileal IMT in our study was reported as lymphoma initially and only on review histopathology and immunohistochemistry was found to be IMT.[12] Yang et al. reported a 56-year-old man with IMT at the left carotid bifurcation accompanied by recurrent syncope and falls. The patient underwent surgery after which corticosteroids and antibiotics were administered for a short-term. No recurrence was observed during the 2-year follow-up.[13,14,15,16] Other unusual presentations include breast lumps, maxillary sinus tumors, renal masses, pemphigus, etc.[17,18,19] Sometimes, they may occur with another clinical condition like sclerosing cholangitis or as a part of double malignancy such as Hodgkin's lymphoma, small cell lung cancer, Wilms tumor, and many others.
The signs and symptoms of IMTs largely depend on the site of involvement.[20,21] Occasionally, they may be completely silent, discovered incidentally on routine imaging or on the evaluation of an inflammatory response at laboratory examination. Grossly, the tumors are circumscribed but not encapsulated and appear as white tan masses with whorled fleshy or myxoid cut surface and may have focal hemorrhage, necrosis, or calcification. Average size is 6 cm. Microscopically these tumors comprise myofibroblastic and fibroblastic spindle cells with inflammatory infiltrate of lymphocytes, plasma cells, eosinophils, and histiocytes against a background of abundant blood vessels. Three patterns have been described. In one pattern, there are elongated myofibroblasts containing abundant eosinophilic cytoplasm and vesicular nuclei, loose myxoid stroma with neutrophils, lymphocytes, and eosinophils, but few plasma cells. Another pattern comprises spindled myofibroblasts and fibroblasts in more compact stroma, arranged as islands surrounded by fibromyxoid stroma with prominent plasma cells and mitotic figures. The third pattern has a densely hyalinized stroma with a few spindle cells; a few plasma cells or lymphocytes. All three patterns have no nuclear pleomorphism and no atypical mitotic figures. Malignant behavior is associated with highly atypical polygonal cells with oval nuclei, prominent nucleoli, Reed–Sternberg-like cells and atypical mitotic figures.[22,23] Approximately 50%–70% of the tumors harbor an ALK gene rearrangement, leading to the formation of a chimeric fusion protein, which is detectable by immunohistochemistry or FISH. ALK is a receptor-type protein tyrosine kinase, which is rendered oncogenic either as a result of a gene fusion, such as in anaplastic large cell lymphoma, lung cancer, and IMT or due to a missense mutation as seen in neuroblastoma and anaplastic thyroid cancer.[24] ALK expression and/or ALK gene rearrangement was previously described as a good prognostic marker in IMT, with positive cases showing a better outcome, whereas ALK-negative IMTs being more aggressive with a higher frequency of metastasis compared with ALK-positive IMT. Previously, due to its markedly variable phenotype and lack of objective immunoprofile, the diagnosis of ALK-negative IMTs has been often a diagnosis of exclusion. In a recent study using next generation sequencing, six of nine ALK-negative IMT tumors showed the presence of fusions in ALK, ROS1, or PDGFRB, suggesting that IMT is largely a kinase fusion – driven neoplasm. The study was initiated by the dramatic response to the ROS1 inhibitor crizotinib in an index case of a treatment-refractory ALK-negative IMT pediatric patient.
There are no clear guidelines regarding the treatment of IMTs. The heterogeneity of these tumors is reflected by the number of treatment options employed by physicians worldwide ranging from surgery to steroids to immunomodulators. Since IMTs usually mimic other tumors in presentation, surgery is usually employed initially with further treatment options followings histopathological diagnosis and immunohistochemistry and cytogenetics. Chemotherapy is recommended when IMT is multifocal, invasive or shows local recurrence.[25,26,27,28] It is often a combination of agents including methotrexate, cisplatin, vinorelbine, Adriamycin, carboplatin, and paclitaxel given with a view of achieving complete remission. Since IMTs involve an inflammatory response, several clinicians have employed nonsteroidal anti-inflammatory agents in their management and found good results.[29] Chaves et al. investigated the anti-inflammatory action of nonsteroidal anti-inflammatory drugs (NSAIDs). The authors analyzed reports of ALK-negative cases receiving NSAID therapy and reported the striking result that NSAIDs were effective in treating ten of 11 cases of IMT. Besides several cases of IMTs have been treated with antibiotics alone. Since Macrolide antibiotics are known to possess unique anti-inflammatory activities, they have been used as a treatment option in the management of IMTs.[30] Watanabe et al. reported a 73-year-old female patient with ALK-negative primary pulmonary IMT who went into remission on treatment with clarithromycin.[31] Similarly, Alezra et al. reported a case of an 8-year-old girl with documented bladder IMT that resolved completely after antibiotic therapy. Since tyrosine kinase mutations are found in IMTs crizotinib has been used by some physicians in the treatment of chemotherapy-resistant disease.
Conclusion
IMT is a unique clinical condition that has confused clinicians and pathologists alike. The disease has a significant diversity in clinical presentation, behavior, histopathology, and treatment options. Although in some cases, the disease may even regress spontaneously, in majority treatment in the form of surgery, chemotherapy, steroids, or immunomodulators is employed; the latter options employed when disease shows local recurrence or distant metastasis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Brunn H. Two interesting benign lung tumors of contradictory histopathology. J Thorac Surg 1939;9:119-31.
- Umiker WO, Iverson L. Postinflammatory tumors of the lung; report of four cases simulating xanthoma, fibroma, or plasma cell tumor. J Thorac Surg 1954;28:55-63.
- Hojo H, Newton WA Jr., Hamoudi AB, Qualman SJ, Wakasa H, Suzuki S, et al. Pseudosarcomatous myofibroblastic tumor of the urinary bladder in children: A study of 11 cases with review of the literature. An Intergroup Rhabdomyosarcoma Study. Am J Surg Pathol 1995;19:1224-36.
- Kenneth A, Jonathan H, Virgil G, Liang C, Edward C, Roxann N, et al. Inflammatory pseudotumor and sarcoma of urinary bladder: Differential diagnosis and outcome in thirty-eight spindle cell neoplasms. Mod Pathol 2001;14:1043-51.
- Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 1995;19:859-72.
- Horn LC, Reuter S, Biesold M. Inflammatory pseudotumor of the ureter and the urinary bladder. Pathol Res Pract 1997;193:607-12.
- Ro JY, el-Naggar AK, Amin MB, Sahin AA, Ordonez NG, Ayala AG. Pseudosarcomatous fibromyxoid tumor of the urinary bladder and prostate: Immunohistochemical, ultrastructural, and DNA flow cytometric analyses of nine cases. Hum Pathol 1993;24:1203-10.
- Roth JA. Reactive pseudosarcomatous response in urinary bladder. Urology 1980;16:635-7.
- Sonobe H, Okada Y, Sudo S, Iwata J, Ohtsuki Y. Inflammatory pseudotumor of the urinary bladder with aberrant expression of cytokeratin. Report of a case with cytologic, immunocytochemical and cytogenetic findings. Acta Cytol 1999;43:257-62.
- Huang WL, Ro JY, Grignon DJ, Swanson D, Ordonez NG, Ayala AG. Postoperative spindle cell nodule of the prostate and bladder. J Urol 1990;143:824-6.
- Oeconomopoulou A, de Verney Y, Kanavaki K, Stefanaki K, Pavlakis K, Salakos C. Inflammatory myofibroblastic tumor of the small intestine mimicking acute appendicitis: A case report and review of the literature. J Med Case Rep 2016;10:100.
- Yang L, Li W, Zhang H. Inflammatory myofibroblastic tumor of carotid artery resulting in recurrent syncope: A case report. Head Neck 2016;38:E2461-3.
- Zhang X, Miao Y, Zhou WW, Xing RG. Inflammatory myofibroblastic tumor in breast: A clinicopathologic study of 2 cases. Zhonghua Bing Li Xue Za Zhi 2016;45:260-1.
- Kim JS, Hong KH, Kim JS, Song JH. Medical therapy of maxillary sinus inflammatory myofibroblastic tumors. Am J Otolaryngol 2016;37:376-8.
- Halpert E, Figueroa JL, Rojas A, Ortiz CI, Chaparro D, Galindo M, et al. Inflammatory myofibroblastic tumor presenting as paraneoplastic pemphigus in a 7-year-old girl. JAAD Case Rep 2016;2:37-40.
- Kapusta LR, Weiss MA, Ramsay J, Lopez-Beltran A, Srigley JR. Inflammatory myofibroblastic tumors of the kidney: A clinicopathologic and immunohistochemical study of 12 cases. Am J Surg Pathol 2003;27:658-66.
- Gough J, Chakrabarti S. Inflammatory pseudotumor of the liver in a patient with chronic sclerosing cholangitis. Am J Gastroenterol 1993;88:1452-3.
- Thomas RM, Jaffe ES, Zarate-Osorno A, Medeiros LJ. Inflammatory pseudotumor of the spleen. A clinicopathologic and immunophenotypic study of eight cases. Arch Pathol Lab Med 1993;117:921-6.
- Newbould MJ, Kelsey A, Lendon M, Gururangan S. Inflammatory pseudotumor of the liver masquerading as a metastasis in a child treated for nephroblastoma. Med Pediatr Oncol 1992;20:172-5.
- Johnson K, Notrica DM, Carpentieri D, Jaroszewski D, Henry MM. Successful treatment of recurrent pediatric inflammatory myofibroblastic tumor in a single patient with a novel chemotherapeutic regimen containing celecoxib. J Pediatr Hematol Oncol 2013;35:414-6.
- Salameh M, Sultan I, Barbar M, Al Hussaini M, Jameel A, Ghandour K, et al. yor causing unexplained anemia in a toddler: A case report. J Med Case Rep 2011;5:69.
- Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: Comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509-20.
- Chan JK, Cheuk W, Shimizu M. Anaplastic lymphoma kinase expression in inflammatory pseudotumors. Am J Surg Pathol 2001;25:761-8.
- Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 2014;4:889-95.
- Choe JY, Go H, Jeon YK, Yun JY, Kim YA, Kim HJ, et al. Inflammatory pseudotumor-like follicular dendritic cell sarcoma of the spleen: A report of six cases with increased IgG4-positive plasma cells. Pathol Int 2013;63:245-51.
- Bosse K, Ott C, Biegner T, Fend F, Siegmann-Luz K, Wallwiener D, et al. 23-year-old female with an inflammatory myofibroblastic tumour of the breast: A case report and a review of the literature. Geburtshilfe Frauenheilkd 2014;74:167-70.
- Bhagat P, Bal A, Das A, Singh N, Singh H. Pulmonary inflammatory myofibroblastic tumor and IgG4-related inflammatory pseudotumor: A diagnostic dilemma. Virchows Arch 2013;463:743-7.
- Tao YL, Wang ZJ, Han JG, Wei P. Inflammatory myofibroblastic tumor successfully treated with chemotherapy and nonsteroidals: A case report. World J Gastroenterol 2012;18:7100-3.
- Chavez C, Hoffman MA. Complete remission of ALK-negative plasma cell granuloma (inflammatory myofibroblastic tumor) of the lung induced by celecoxib: A case report and review of the literature. Oncol Lett 2013;5:1672-6.
- Watanabe H, Uruma T, Tazaki G, Tajiri T, Kikuchi R, Itoh M, et al. Remission of ALK-negative primary pulmonary inflammatory myofibroblastic tumor on treatment with clarithromycin: A case report and review of the literature. Oncol Lett 2016;11:1757-61.
- Alezra E, Delforge X, Buisson P, Gourmel A, Cordonnier C, Ricard J, et al. Complete resolution of inflammatory myofibroblastic tumor of the bladder after antibiotic therapy. Arch Pediatr 2016;23:612-5.

| Figure 1:Contrast-enhanced computed tomography of abdomen and pelvis revealed a 7 cm × 5 cm × 5 cm lobulated hyper enhancing mass lesion with central necrosis arising from bowel wall extraluminally in ileocecal area suggestive of gut lymphoma

| Figure 2:Low power view of Histopathological examination (HPE) showing spindle cells with inflammatory background of plasma cells, lymphocytes, and neutrophils

| Figure 3:High power view of HPE showing spindle cells with inflammatory background of plasma cells lymphocytes and neutrophils
References
- Brunn H. Two interesting benign lung tumors of contradictory histopathology. J Thorac Surg 1939;9:119-31.
- Umiker WO, Iverson L. Postinflammatory tumors of the lung; report of four cases simulating xanthoma, fibroma, or plasma cell tumor. J Thorac Surg 1954;28:55-63.
- Hojo H, Newton WA Jr., Hamoudi AB, Qualman SJ, Wakasa H, Suzuki S, et al. Pseudosarcomatous myofibroblastic tumor of the urinary bladder in children: A study of 11 cases with review of the literature. An Intergroup Rhabdomyosarcoma Study. Am J Surg Pathol 1995;19:1224-36.
- Kenneth A, Jonathan H, Virgil G, Liang C, Edward C, Roxann N, et al. Inflammatory pseudotumor and sarcoma of urinary bladder: Differential diagnosis and outcome in thirty-eight spindle cell neoplasms. Mod Pathol 2001;14:1043-51.
- Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 1995;19:859-72.
- Horn LC, Reuter S, Biesold M. Inflammatory pseudotumor of the ureter and the urinary bladder. Pathol Res Pract 1997;193:607-12.
- Ro JY, el-Naggar AK, Amin MB, Sahin AA, Ordonez NG, Ayala AG. Pseudosarcomatous fibromyxoid tumor of the urinary bladder and prostate: Immunohistochemical, ultrastructural, and DNA flow cytometric analyses of nine cases. Hum Pathol 1993;24:1203-10.
- Roth JA. Reactive pseudosarcomatous response in urinary bladder. Urology 1980;16:635-7.
- Sonobe H, Okada Y, Sudo S, Iwata J, Ohtsuki Y. Inflammatory pseudotumor of the urinary bladder with aberrant expression of cytokeratin. Report of a case with cytologic, immunocytochemical and cytogenetic findings. Acta Cytol 1999;43:257-62.
- Huang WL, Ro JY, Grignon DJ, Swanson D, Ordonez NG, Ayala AG. Postoperative spindle cell nodule of the prostate and bladder. J Urol 1990;143:824-6.
- Oeconomopoulou A, de Verney Y, Kanavaki K, Stefanaki K, Pavlakis K, Salakos C. Inflammatory myofibroblastic tumor of the small intestine mimicking acute appendicitis: A case report and review of the literature. J Med Case Rep 2016;10:100.
- Yang L, Li W, Zhang H. Inflammatory myofibroblastic tumor of carotid artery resulting in recurrent syncope: A case report. Head Neck 2016;38:E2461-3.
- Zhang X, Miao Y, Zhou WW, Xing RG. Inflammatory myofibroblastic tumor in breast: A clinicopathologic study of 2 cases. Zhonghua Bing Li Xue Za Zhi 2016;45:260-1.
- Kim JS, Hong KH, Kim JS, Song JH. Medical therapy of maxillary sinus inflammatory myofibroblastic tumors. Am J Otolaryngol 2016;37:376-8.
- Halpert E, Figueroa JL, Rojas A, Ortiz CI, Chaparro D, Galindo M, et al. Inflammatory myofibroblastic tumor presenting as paraneoplastic pemphigus in a 7-year-old girl. JAAD Case Rep 2016;2:37-40.
- Kapusta LR, Weiss MA, Ramsay J, Lopez-Beltran A, Srigley JR. Inflammatory myofibroblastic tumors of the kidney: A clinicopathologic and immunohistochemical study of 12 cases. Am J Surg Pathol 2003;27:658-66.
- Gough J, Chakrabarti S. Inflammatory pseudotumor of the liver in a patient with chronic sclerosing cholangitis. Am J Gastroenterol 1993;88:1452-3.
- Thomas RM, Jaffe ES, Zarate-Osorno A, Medeiros LJ. Inflammatory pseudotumor of the spleen. A clinicopathologic and immunophenotypic study of eight cases. Arch Pathol Lab Med 1993;117:921-6.
- Newbould MJ, Kelsey A, Lendon M, Gururangan S. Inflammatory pseudotumor of the liver masquerading as a metastasis in a child treated for nephroblastoma. Med Pediatr Oncol 1992;20:172-5.
- Johnson K, Notrica DM, Carpentieri D, Jaroszewski D, Henry MM. Successful treatment of recurrent pediatric inflammatory myofibroblastic tumor in a single patient with a novel chemotherapeutic regimen containing celecoxib. J Pediatr Hematol Oncol 2013;35:414-6.
- Salameh M, Sultan I, Barbar M, Al Hussaini M, Jameel A, Ghandour K, et al. yor causing unexplained anemia in a toddler: A case report. J Med Case Rep 2011;5:69.
- Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: Comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509-20.
- Chan JK, Cheuk W, Shimizu M. Anaplastic lymphoma kinase expression in inflammatory pseudotumors. Am J Surg Pathol 2001;25:761-8.
- Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 2014;4:889-95.
- Choe JY, Go H, Jeon YK, Yun JY, Kim YA, Kim HJ, et al. Inflammatory pseudotumor-like follicular dendritic cell sarcoma of the spleen: A report of six cases with increased IgG4-positive plasma cells. Pathol Int 2013;63:245-51.
- Bosse K, Ott C, Biegner T, Fend F, Siegmann-Luz K, Wallwiener D, et al. 23-year-old female with an inflammatory myofibroblastic tumour of the breast: A case report and a review of the literature. Geburtshilfe Frauenheilkd 2014;74:167-70.
- Bhagat P, Bal A, Das A, Singh N, Singh H. Pulmonary inflammatory myofibroblastic tumor and IgG4-related inflammatory pseudotumor: A diagnostic dilemma. Virchows Arch 2013;463:743-7.
- Tao YL, Wang ZJ, Han JG, Wei P. Inflammatory myofibroblastic tumor successfully treated with chemotherapy and nonsteroidals: A case report. World J Gastroenterol 2012;18:7100-3.
- Chavez C, Hoffman MA. Complete remission of ALK-negative plasma cell granuloma (inflammatory myofibroblastic tumor) of the lung induced by celecoxib: A case report and review of the literature. Oncol Lett 2013;5:1672-6.
- Watanabe H, Uruma T, Tazaki G, Tajiri T, Kikuchi R, Itoh M, et al. Remission of ALK-negative primary pulmonary inflammatory myofibroblastic tumor on treatment with clarithromycin: A case report and review of the literature. Oncol Lett 2016;11:1757-61.
- Alezra E, Delforge X, Buisson P, Gourmel A, Cordonnier C, Ricard J, et al. Complete resolution of inflammatory myofibroblastic tumor of the bladder after antibiotic therapy. Arch Pediatr 2016;23:612-5.


 PDF
PDF  Views
Views  Share
Share

