Clinical profile and outcome of adult Hodgkin lymphoma: Experience from a tertiary care institution
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(04): 255-260
DOI: DOI: 10.4103/0971-5851.171550
Abstract
Treatment and outcome of Hodgkin lymphoma (HL) are the true success story of modern medicine. The data from the developing countries on long-term outcome of patients with HL is sparse. Aims: Primary objective is to assess the progression-free survival (PFS). Secondary objective are overall survival (OS) and toxicities. Settings and Design: This is a retrospective analysis from the case records from a single institution. Materials and Methods: Institutional Ethical Committee approval was obtained. Between January 1991 and December 2010, 301 patients (age ≥18 years) underwent treatment at our institution. Statistical Analysis: Kaplan-Meyer curves were used to calculate the PFS and OS. Results: The median age at presentation was 36 years, range from 19 to 75 years. The male to female ratio was 2.9:1. Seventy-five percent of patients had B symptoms. Majority presented in advanced stage (Stage III and IV) disease (64.7%). Mixed cellularity (74.4%) was the most common histology, followed by nodular sclerosis (13.9%). The most common chemotherapy regimen used was ABVD (61%). Conclusions: Median follow-up of the cohort was 18.5 months (range 2-225). PFS and OS rate at 5 years is 66.3% and 79.7% respectively.
Publication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Treatment and outcome of Hodgkin lymphoma (HL) are the true success story of modern medicine. The data from the developing countries on long-term outcome of patients with HL is sparse.
Aims:
Primary objective is to assess the progression-free survival (PFS). Secondary objective are overall survival (OS) and toxicities.
Settings and Design:
This is a retrospective analysis from the case records from a single institution.
Materials and Methods:
Institutional Ethical Committee approval was obtained. Between January 1991 and December 2010, 301 patients (age ≥18 years) underwent treatment at our institution.
Statistical Analysis:
Kaplan–Meyer curves were used to calculate the PFS and OS.
Results:
The median age at presentation was 36 years, range from 19 to 75 years. The male to female ratio was 2.9:1. Seventy-five percent of patients had B symptoms. Majority presented in advanced stage (Stage III and IV) disease (64.7%). Mixed cellularity (74.4%) was the most common histology, followed by nodular sclerosis (13.9%). The most common chemotherapy regimen used was ABVD (61%).
Conclusions:
Median follow-up of the cohort was 18.5 months (range 2-225). PFS and OS rate at 5 years is 66.3% and 79.7% respectively.
INTRODUCTION
Treatment and outcome of Hodgkin lymphoma (HL) are the true success story of modern medicine. There was a gradual improvement in the outcome of HL with the advent of better understanding of the biology of disease, staging techniques, treatment involving multidiscipline's of modern medicine and tailoring of treatment.[1,2,3,4] The outcome of HL in developed countries from the cancer registries are widely available,[5,6,7] few studies with small number of the patient are reported from the Indian subcontinent.[8,9,10,11,12,13,14,15,16] This is the first study from the state of Andhra Pradesh, attempting to add data to the existing literature. We have analyzed HL patients treated at our center over a 20-year period.
MATERIALS AND METHODS
This is a retrospective analysis of HL presenting to a single tertiary care center in the region. Between January 1991 and December 2010, 370 patient's aged more than 18yrs who were newly diagnosed. Of these, 301 underwent complete work-up and received at least 2 cycles of treatment and were analyzed. The initial work-up included detailed clinical evaluation (history and physical examination), complete blood counts, viral markers, two-dimensional echo (if indicated), renal and liver function tests, and serum lactate dehydrogenase. Lymph node (LN) biopsy was done in all patients. Computed tomography (CT) scan or ultrasound of the whole abdomen, CT scan or X-ray of the chest or positron emission tomography-CT whole body. Except in Stage I patients, bone marrow biopsies were done in all other patients. Patients were staged according to the modified Ann Arbor system.[3,17] A diagnosis of HL was made based on morphological features on the LN biopsy with further confirmation by immunohistochemistry and sub-classification as per the WHO criteria.[18] A mediastinal mass occupying more than one-third of the thoracic diameter or any LN mass more than 10 cm was taken as a bulky disease. All the patients were consented for treatment before starting chemotherapy. Institutional ethical committee approval was taken before analyzing the data.
Treatment protocol
A few patients before 1999 received MOPP, COPP/ABV or COPP-type regimens, and standard ABVD chemotherapy was administered in remaining patients. The doses of ABVD are already described.[16] Patients with early stage HL (Stages IA, IB, and IIA) were planned for 4 cycles of ABVD chemotherapy followed by involved field radiotherapy (IFRT 25-40 Gy). All the other patients were planned for 6-8 cycles of chemotherapy with radiotherapy (RT) for initial bulky sites and/or residual disease. Patients failing first-line therapy were offered second-line chemotherapy ± RT.
Response assessment
Patients were examined prior to each dose of chemotherapy for clinical response and toxicity. The response was assessed by both clinical and radiological at the end of 3-4 cycles of chemotherapy and then at the end of therapy. Complete response (CR) was defined as complete resolution of disease at all sites clinically and radiologically and normal bone marrow studies (wherever involved at diagnosis). Partial response (PR) was defined as a decrease in diameter of measurable lesions by ≥50%. Progressive disease (PD) as the appearance of new lesions or increase in the size of lesions. Stable disease (SD) if does not fit into above two criteria.[19]
Follow-up
Patients in complete remission were followed 3 months for the first 2 years, and 6 months for next 3 years and yearly thereafter.
Statistical analysis
Progression-free survival (PFS) was defined as time from start of treatment till relapse or PD, no CR at the end of first-line treatment, or death due to any cause, loss to follow-up with disease. Overall survival (OS) was defined as the time from the diagnosis to the death, loss to follow-up with the disease. Patient characteristics were analyzed using descriptive statistics. Inferential statistics was utilized for prognostic factors using log-rank t-test and the Kaplan–Meier curve, estimated survival. GraphPad prism 4.0 (GraphPad Software Inc, California, USA) is used for the survival curves and prognostic factors.
RESULTS
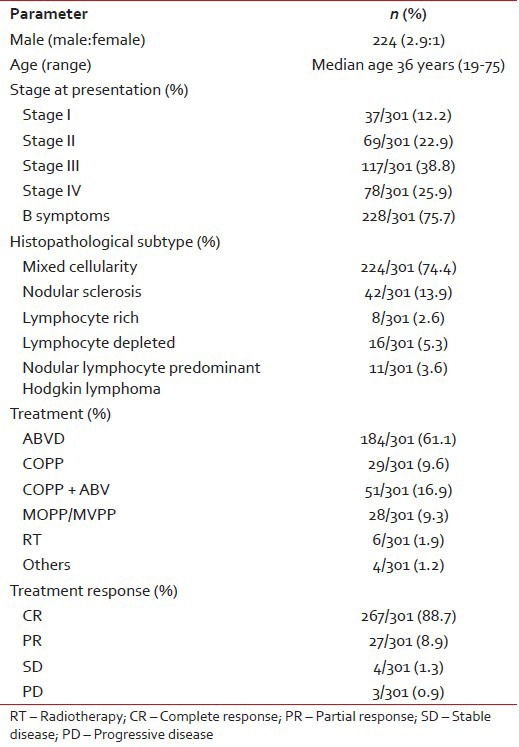
Patient characteristics are given in Table 1. A total of 301 patients were eligible for analysis. The median age of presentation was 36 years (range 19-75). There were of a total of 224 males and 77 females (ratio 2.9:1). Two hundred and twenty-eight (75.7%) patients presented with B symptoms. Staging with standard investigations revealed 37/301 (12.2%), 69/301 (22.9%), 117/301 (38.8%) 78/301 (25.9%) as Stage I, II, III and IV respectively. Fifty-five (18.2%) patients had the bulky disease at initial presentation. The histopathological examination revealed mixed cellularity (MC) in 224/301 (74.4%) of patients, followed by nodular sclerosis (NS) in 42/301 (13.9%), lymphocyte rich in 8/301 (2.6%), lymphocyte depleted in 16/301 (5.3%). Eleven patients (3.6%) had nodular lymphocyte predominant HL.
Table 1
Patient characteristics (n = 301)
Treatment
Chemotherapy
The standard ABVD every 2 weekly was given in 184/301 (61.1%) as first-line treatment. The hybrid regimen COPP + ABV were given in 51/301 (16.9%). First generation protocol MOPP/MVPP in 28/301 (9.3%) and 29/301 (9.6%) received COPP.
Radiotherapy
Among those treated with chemotherapy, 12.2% received IFRT. Six patients (all Stage IA) received RT alone. The RT doses ranged from 20 to 45 Gy.
Outcome
Response to treatment
At end of the planned first line treatment the response to treatment were evaluated, 267/301 (81%) attained CR, 27/301 (8.9%) had PR, 4/301 (1.3%) had SD and 3/301 (0.9%) had PD. The median follow-up was 18.5 months (range 2-225). The median PFS has not reached yet. The 5 years PFS was 66.3% [Figure 1]. The median OS for the entire cohort has not reached. The OS at 5 years is 79.7%.
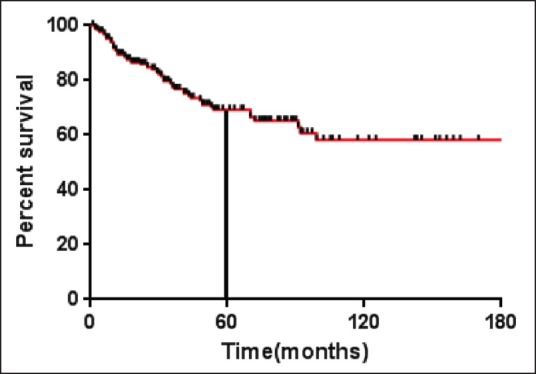
| Fig. 1 Progression free survival (months) for the entire cohort (n = 301)
Prognostic factors
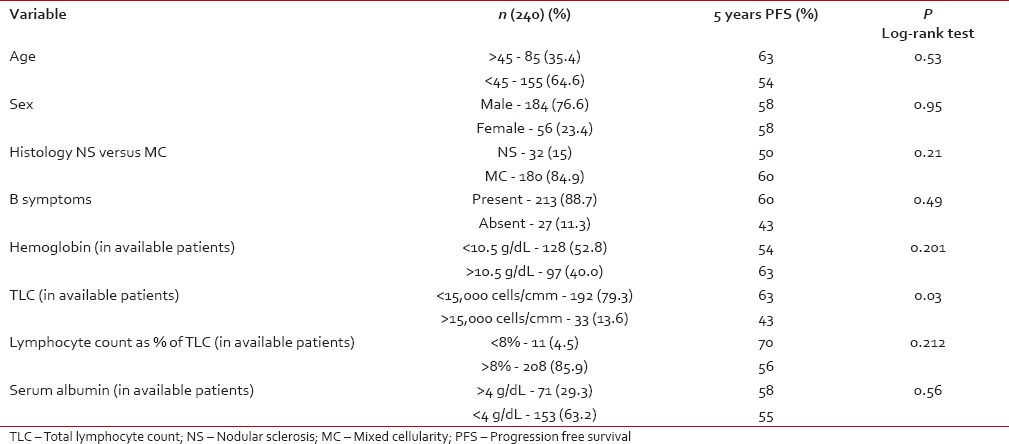
Stage at diagnosis had significant impact on the PFS, the PFS at 5 years was significantly higher for patients with early stage (Stage IA, IB, IIA) (80.5%) compared to advanced HL (IIB, IIIA, IIIB, IVA, IVB) (65.7%) (P = 0.005) [Figure 2]. For the whole group, there was a trend for better PFS for patients without B symptoms (P = 0.09). Other factors like age at diagnosis, gender, histology, hemoglobin, total leukocyte count, absolute lymphocyte count, and serum albumin had no influence on the PFS [Table 2].
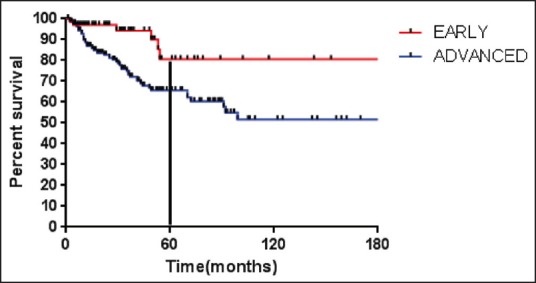
| Fig. 2 Progression free survival (months) for early and advanced stage
Table 2
Prognostic factors (all patients included)
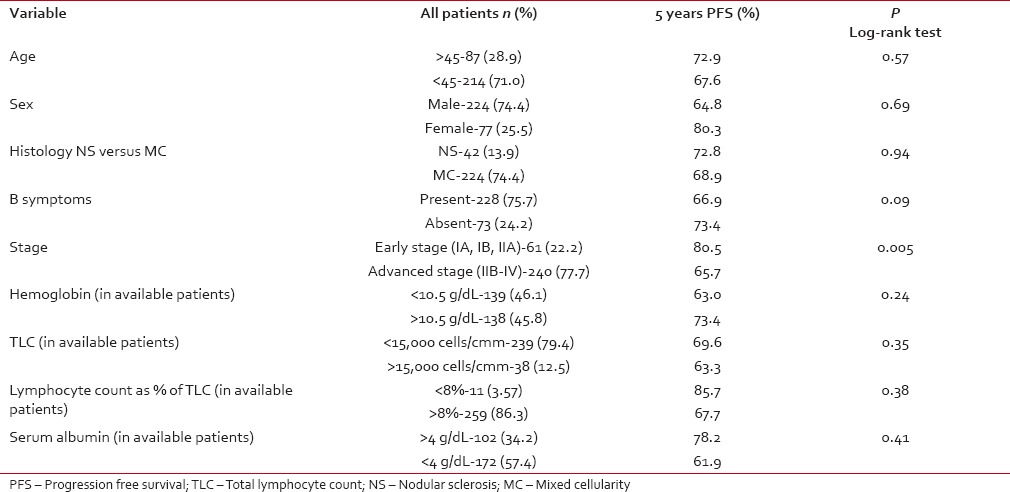 In advanced stage disease (IIB-IVB), total leukocyte count at the time of diagnosis had statistical significance for PFS. Other factors like age at diagnosis, gender, histology, hemoglobin, absolute lymphocyte count, and serum albumin did not influence PFS [Table 3].
In advanced stage disease (IIB-IVB), total leukocyte count at the time of diagnosis had statistical significance for PFS. Other factors like age at diagnosis, gender, histology, hemoglobin, absolute lymphocyte count, and serum albumin did not influence PFS [Table 3].Table 3
Advanced stage (Stage IIB-IVB) prognostic factors
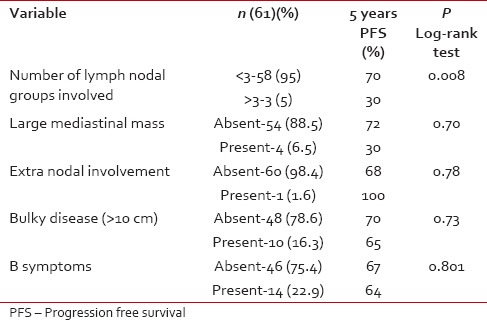
 In the early stage (IA, IB, IIA), there was significant PFS benefit for patients with less than three LN improvement. There was no effect of size of the mediastinal mass, extranodal involvement, bulky disease, and B symptoms on PFS [Table 4].
In the early stage (IA, IB, IIA), there was significant PFS benefit for patients with less than three LN improvement. There was no effect of size of the mediastinal mass, extranodal involvement, bulky disease, and B symptoms on PFS [Table 4].Table 4
Early stage (IA/B and IIA)
Toxicities and complications
Grades 3-4 myelosuppression was the most common hematological toxicity with or without febrile neutropenia in 37/301 (12.2%). Other toxicities were anemia, thrombocytopenia, nausea and vomiting, neurotoxicity, pulmonary, and cardiac [Table 5].
Table 5
Toxicities and complications

DISCUSSION
This retrospective analysis gives us a broad overview about the real world scenario of the cancer treatment facility in the state of Andhra Pradesh. To the best of our knowledge at least 15-20% of patients refuse treatment, the reason could be low socioeconomic status, illiteracy, lack of awareness, distance from the treatment center and moving to better treatment center. At least 20-25% of patients were lost to follow-up at different time points, the reasons could be a relapse, change of address, employment, and others. Since 2008 onwards, the Government of Andhra Pradesh took an initiative to provide a health insurance scheme for below poverty line, this may improve treatment outcomes.
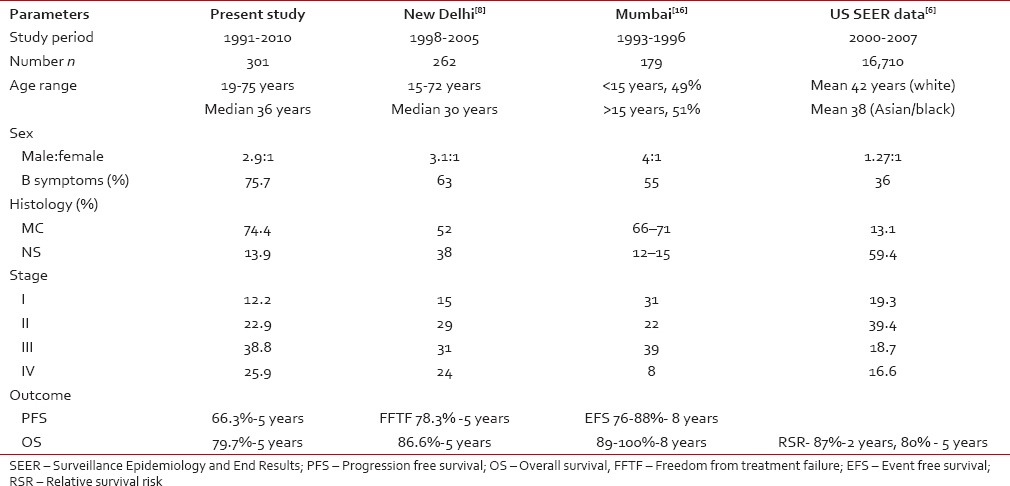
Comparisons between the different studies are given in Table 6. There are two distinct features from this study, the median age of presentation is lesser than the western countries, and there is also the absence of a bimodal distribution, which was also seen in other Indian studies.[8,13] A similar pattern is also seen in the Asian and Blacks in SEER data.[6] Other findings are higher M to F ratio, higher frequency of B symptoms, a higher proportion of patients with advanced HL, and MC as the predominant histology subtype. The peak incidence of disease was in the fourth decade, and there was no second peak after 50 years. Median age is slightly higher than in other studies as we have included patients from 19 years, and many Indian studies have included from 15 years.[8,16] Higher male to female ratio has also been reported in earlier studies from India[8,9,10,11,12,13,14,15,16] and other countries with limited resources.[20] In the present study, the clinical presentation is more or less similar to earlier studies. Here, notably 75.7% of patients had B symptoms at presentation. This is higher than in the studies from developed countries (≤20%).[3,4,5,6,7] Similarly, a higher proportion of patients (64.7%) had Stages III and IV disease, a figure higher than that reported in US SEER data-36%.[6] These adverse prognostic features at initial presentation in the present study possibly could be due to delay in presentation. Seventy-four percent of patients had MC histology, a figure similar to reports from countries with limited resources,[20] but much higher than that reported from a developed nations where NS subtype is common[3,4,5,6,7] [Table 6].
Table 6
Comparative studies
 In the present study, the patients advanced stage (IIB-IV vs. I-II), is a significant predictor of PFS. Other variable like age, sex, total leukocyte count, absolute lymphocyte count, low hemoglobin, B symptoms, and bulk disease were not predictors for PFS.
In the present study, the patients advanced stage (IIB-IV vs. I-II), is a significant predictor of PFS. Other variable like age, sex, total leukocyte count, absolute lymphocyte count, low hemoglobin, B symptoms, and bulk disease were not predictors for PFS.In the early stage (IA, IB, IIA) there is significant PFS benefit for patients with less than three LN improvement. In advanced stage disease (IIB-IVB) total leukocyte count at the time of diagnosis has statistical significance for PFS. Other factors like age at diagnosis, gender, histology, hemoglobin, absolute lymphocyte count, and serum albumin had no influence on PFS. The reasons could be small patient numbers, short follow-up period, inclusion of loss to follow-up with the disease as event and selection bias.
The PFS and OS are marginally inferior to the studies from India, as we have included a loss to follow-up with the disease in our present analysis.[8,13,16] Given the overall circumstances the outcome of HL is encouraging in this part of the country.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments

| Fig. 1 Progression free survival (months) for the entire cohort (n = 301)

| Fig. 2 Progression free survival (months) for early and advanced stage


 PDF
PDF  Views
Views  Share
Share

