Clinical Benefit and Safety of Palbociclib in Hormone Positive Breast Cancer with Visceral Metastasis: Real-World Experience from a Tertiary Cancer Center
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(02): 168-173
DOI: DOI: 10.1055/s-0044-1790584
Abstract
Introduction Palbociclib, the first CDK4/6 inhibitor, has shown promising results in phase III clinical studies by enhancing the efficacy of endocrine therapy (ET) in HR +/HER2– advanced breast cancer. However, real-world data on its use in patients with visceral metastatic disease are limited. We aimed to assess the effectiveness and tolerability of palbociclib in this high-risk population across different lines of treatment.
Materials and Methods Patients with hormone-positive metastatic breast cancer who received palbociclib with ET between 2015 and 2021 were grouped into skeletal and visceral metastatic disease. Visceral metastatic diseases were subclassified into lung, liver, and brain metastatic diseases. All subgroups were analyzed for progression-free survival (PFS), toxicity, and prognostic factors. Subgroups were compared using the chi-square test, and survival analyses were done using the Kaplan–Meier test.
Results Among 100 patients who received palbociclib, 70 had progressed on previous ET. The common metastatic site was bone (56%), followed by lung (24%), liver (18%), and brain (2%). With a median follow-up of 37 months, the median PFS of the overall population was 24 months: bone metastasis 27 months, lung 25 months, liver 12 months, and brain 4 months. Weak hormone positivity, ET-resistant metastatic patients, and high grade were associated with poorer responses. The common side effects were neutropenia (40%), anemia (35%), thrombocytopenia (15%), and hepatotoxicity (10%). Three percent of patients discontinued treatment due to toxicity.
Conclusion Palbociclib with ET showed improved PFS and safety in visceral metastatic disease, comparable to randomized controlled trials. However, further studies are required to evaluate its efficacy in extensive visceral metastatic disease and previously heavily treated patients.
Keywords
CDK4/6 inhibitors - palbociclib - visceral metastatic breast cancer - metastatic hormone-positive breast cancerPatient Consent
Informed patient consent was obtained to conduct this study.
Publication History
Article published online:
17 September 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Challenges in the Management of Lung Cancer: Real-World Experience from a Tertiary Center in South IndiaVishnu Gopal, South Asian Journal of Cancer, 2021
- Real-World Experience of Treating Young Adult Patients with Breast Cancer from a Single Center in Southern IndiaPriya Iyer, South Asian Journal of Cancer
- Clinicopathological Evaluation of Patients with Hormone Receptor–Positive HER2-Negative Metastatic Breast Cancer Progressing on Endocrine Treatment: A Real-Worl...S. Shanthala, et al., South Asian Journal of Cancer, 2023
- Real-World Outcome of Platinum-Based Chemotherapy in Advanced Breast Cancer (ABC): A Retrospective Study from a Tertiary Cancer Center in IndiaIndhuja Muthiah Vaikundaraja, Indian J Radiol Imaging, 2021
- Real-World Experience of AI-Assisted Endocytoscopy Using EndoBRAIN—An Observational Study from a Tertiary Care CenterAnudeep Katrevula, Journal of Digestive Endoscopy
- REAL WORLD EFFECTIVENESS AND SAFETY OF OZANIMOD: INITIAL RESULTS FROM A LARGE TERTIARY CENTER<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Tofacitinib in Acute Severe Ulcerative Colitis—A Real-World Tertiary Center Experience<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Real-World Experience with CDK4/6 Inhibitors for Metastatic HR+/HER2− Breast Cancer at a Single Cancer Center<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- P562 Real world effectiveness and safety of ozanimod: One-year follow-up from a large tertiary center<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Real-World Effectiveness of Palbociclib Plus Aromatase Inhibitors in African American Patients With Metastatic Breast Cancer<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
Abstract
Introduction Palbociclib, the first CDK4/6 inhibitor, has shown promising results in phase III clinical studies by enhancing the efficacy of endocrine therapy (ET) in HR +/HER2– advanced breast cancer. However, real-world data on its use in patients with visceral metastatic disease are limited. We aimed to assess the effectiveness and tolerability of palbociclib in this high-risk population across different lines of treatment.
Materials and Methods Patients with hormone-positive metastatic breast cancer who received palbociclib with ET between 2015 and 2021 were grouped into skeletal and visceral metastatic disease. Visceral metastatic diseases were subclassified into lung, liver, and brain metastatic diseases. All subgroups were analyzed for progression-free survival (PFS), toxicity, and prognostic factors. Subgroups were compared using the chi-square test, and survival analyses were done using the Kaplan–Meier test.
Results Among 100 patients who received palbociclib, 70 had progressed on previous ET. The common metastatic site was bone (56%), followed by lung (24%), liver (18%), and brain (2%). With a median follow-up of 37 months, the median PFS of the overall population was 24 months: bone metastasis 27 months, lung 25 months, liver 12 months, and brain 4 months. Weak hormone positivity, ET-resistant metastatic patients, and high grade were associated with poorer responses. The common side effects were neutropenia (40%), anemia (35%), thrombocytopenia (15%), and hepatotoxicity (10%). Three percent of patients discontinued treatment due to toxicity.
Conclusion Palbociclib with ET showed improved PFS and safety in visceral metastatic disease, comparable to randomized controlled trials. However, further studies are required to evaluate its efficacy in extensive visceral metastatic disease and previously heavily treated patients.
Keywords
CDK4/6 inhibitors - palbociclib - visceral metastatic breast cancer - metastatic hormone-positive breast cancerIntroduction
Breast cancer is the most commonly observed cancer (13.5%-of total cases) and the leading cause of cancer death (10.6%-of total cases) in India, contributing significantly to the cancer burden.[1] Hormone receptor-positive subtypes are the most common, both in India and worldwide, with an incidence in India ranging from 25 to 60%.[2] [3] [4]
The pathogenesis in the hormone receptor-positive subgroup is driven by estradiol through the estrogen receptor/progesterone receptor (ER/PR) pathway.[5] These subgroups are considered prognostically better; hence, hormonal agents are the treatment of choice in metastatic breast cancer (MBC) unless the patient has a visceral crisis or progressive visceral metastasis.[6] [7]
Six years have elapsed since the first cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitor was approved for use in ER+ breast cancer. Its effectiveness and safety when paired with endocrine therapy (ET) have been established through numerous randomized trials, making it the preferred first-line treatment for the majority of patients[8]
Palbociclib, a first-in-class, selective inhibitor of CDK4/6, when combined with ET, results in synergistic effects. These findings led to the design of clinical studies in which the addition of palbociclib to ET resulted in significantly improved progression-free survival (PFS) in both previously treated (PALOMA-3)[9] and treatment-naive (PALOMA-1 and -2)[8] [10] women with HR +/HER2– advanced breast cancer.
The real-world experience of patients with visceral metastatic disease, prognostic factors, and tolerance of this drug in the first, second, and subsequent lines is limited. It is crucial to consider the potential outcomes and risks for patients with visceral organ metastases, who are at high risk, when exploring new treatment options.
In our study, we analyzed the real-world efficacy and tolerance of palbociclib across different subgroups and treatment lines among HR+ patients with MBC affecting visceral organs.
Materials and Methods
Objectives
Evaluate real-world data for PFS and objective response rate palbociclib in HR + ve MBC with visceral metastatic disease. Tolerance and toxicity of palbociclib in visceral metastatic disease. Compare with the randomized controlled trial (RCT) and other real-world evidence.
Methods
In our present retrospective single-institutional observational study, after obtaining ethical committee approval, we documented clinical, demographic, and tumor-related information, as well as treatment-related toxicity details of patients with hormone-positive HER2 negative MBC who received palbociclib in combination with ET between 2015 and 2021. Patients with a minimum of 2 years of follow-up were analyzed for PFS. Patients were grouped into skeletal and visceral metastatic disease. Visceral metastatic diseases were subclassified into lung, liver, and brain metastatic diseases, while all subgroups were assessed for PFS, toxicity, and prognostic factors.
Primary outcome: Median PFS of HR+ MBC patients treated with palbociclib and ET, stratified by metastatic site (skeletal vs. visceral) and further subclassified into lung, liver, and brain metastases.
Secondary outcomes: Prognostic factors influencing treatment response, including hormone receptor status, ET menopausal status, different lines of palbociclib use, and tumor grade. Evaluation of the safety profile of palbociclib in real-world clinical practice, focusing on the incidence of toxicities, dose reduction, and treatment discontinuation due to toxicity.
Comparison of PFS between different subgroups using the chi-square test and survival analysis with the Kaplan–Meier method.
Assessment of the overall clinical benefit and safety of palbociclib in HR+ MBC with visceral metastasis, including its comparability to results from RCTs.
Inclusion criteria: Hormone receptor-positive MBC patients who have been treated with palbociclib in combination with ET between 2015 and 2021.
Exclusion criteria: Patients with severe comorbidities, those lost to follow-up, individuals who discontinued palbociclib for reasons other than disease progression or intolerance, those with multiple malignancies, and individuals experiencing visceral crisis.
Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 20. All subgroups were analyzed for PFS, toxicity, and prognostic factors. Subgroups were compared using the chi-square test. Survival times and rates were evaluated with the Kaplan–Meier method. Median overall survival (OS) was calculated from the day of starting palbociclib. Factors affecting the treatment results were evaluated using log-rank and Cox regression tests. A p-value of less than 0.05 was considered statistically significant.
Ethics
In accordance with the ethical principles outlined in the Declaration of Helsinki, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Institutional Ethical Committee Sri Shankara Cancer Hospital and Research Centre IEC approval no: SSRHRC/IEC16/131; Date of approval: 12-11-2022.
Results
Patient Demographic Details
A total of 100 patients who received palbociclib between 2016 and 2021 were analyzed, with a median follow-up duration of 37 months. The median age of the population was 56 years. Among these patients, 24%-were premenopausal, 30%-had progressed on previous hormonal therapy, and 70%-had metastatic disease without prior hormonal therapy at presentation. Fifty-six percent of the patients had only skeletal metastasis, while 44%-had visceral metastasis, with no patients experiencing a visceral crisis. Specifically, 24%-had lung metastasis, 18%-had liver metastasis, and 2%-had brain metastasis ([Table 1]).
|
CDK4/6 inhibitors |
Palbociclib |
Percentage |
|---|---|---|
|
Age group |
||
|
> 60 |
43 |
43 |
|
50–59 |
33 |
33 |
|
40–49 |
15 |
15 |
|
< 40> |
9 |
9 |
|
Menstruation |
||
|
Premenopause |
24 |
24 |
|
Postmenopause |
76 |
76 |
|
Presentation |
||
|
ET resistant |
30 |
30 |
|
De novo metastasis |
70 |
70 |
|
Metastatic sites |
||
|
Skeletal |
56 |
56 |
|
Visceral metastasis |
44 |
44 |
|
CDK4/6 inhibitors |
Palbociclib |
|---|---|
|
Histopathology |
|
|
Intraductal |
86 |
|
Lobular |
8 |
|
Other |
6 |
|
Grade |
|
|
1 |
8 |
|
2 |
56 |
|
3 |
36 |
|
Hormone status |
|
|
Strong positive |
67 |
|
Weak positive |
33 |
|
HER 2NEU |
|
|
Negative |
89 |
|
Low expression |
10 |
|
Positive |
1 |
|
KI-67% |
|
|
< 15> |
26 |
|
15–29% |
22 |
|
> 30 |
52 |
|
Sequence |
No. of patients |
|---|---|
|
First line |
51 |
|
Second line |
37 |
|
Third and subsequent |
12 |
|
CDKi + Endo |
|
|
CDKi + AI |
55 |
|
CDKi + Fulvestrant |
42 |
|
CDKi + Exemestane |
3 |
|
Toxicities |
|
|
No AE |
30 |
|
Anemia (grade I II) |
35 |
|
Neutropenia (I II) |
40 |
|
Thrombocytopenia (grade I II) |
15 |
|
Nonhematological |
22 |
|
Hematological (grade III IV) |
3 |
|
Nonhematological (grade III IV) |
2 |
|
Dose modification |
|
|
No modification |
64 |
|
First dose |
25 |
|
Second dose |
8 |
|
Discontinue |
3 |
|
Response to treatment |
|
|
Complete |
5 |
|
Partial |
41 |
|
Progress |
47 |
|
Death |
7 |
Treatment Outcomes
Among the patients treated with palbociclib, 5%-exhibited a complete response, 41%-showed a partial response, and 47%-experienced disease progression. Additionally, 7 patients died during treatment. The median PFS of palbociclib when used in the first line was 32 months, compared to 23 months in the second line, and 13 months in the third line or subsequent lines. Premenopausal women had a PFS of 21 months compared to 25 months for postmenopausal women.
Weak hormone positivity, ET-resistant metastatic patients, and high grade were identified as poor responders. With a median follow-up of 37 months, the median PFS of the overall population was 24 months. Specifically, bone metastasis had a median PFS of 27 months, lung metastasis 25 months, liver metastasis 12 months, and brain metastasis 4 months ([Table 4]; ([Graphs 1] and [2]).
|
Median PFS in subgroups |
Months |
p-Value |
|---|---|---|
|
Sequence of palbociclib |
||
|
First line |
32 |
|
|
Second line |
23 |
0.170 |
|
Third and more |
13 |
|
|
Menstrual status |
||
|
Premenopausal |
21 |
|
|
Postmenopausal |
25 |
0.218 |
|
Site of metastasis |
||
|
Skeletal |
27 |
|
|
Visceral |
15 |
0.054 |
|
Hormonal status |
||
|
Strong positive |
32 |
0.127 |
|
Weak positive |
14 |
|
|
KI-67 |
||
|
< 15> |
40 |
0.189 |
|
15–30 |
19 |
|
|
> 30 |
22 |
|
|
Presentation |
||
|
ET resistant |
22 |
|
|
De novo |
40 |
0.113 |
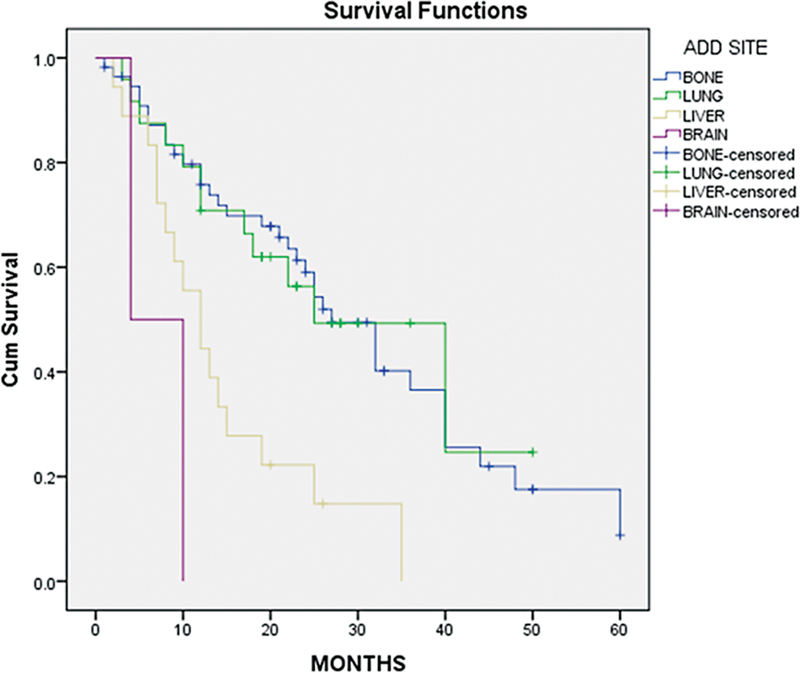
| Fig 1 : Progression-free survival (PFS) based on metastatic sites.
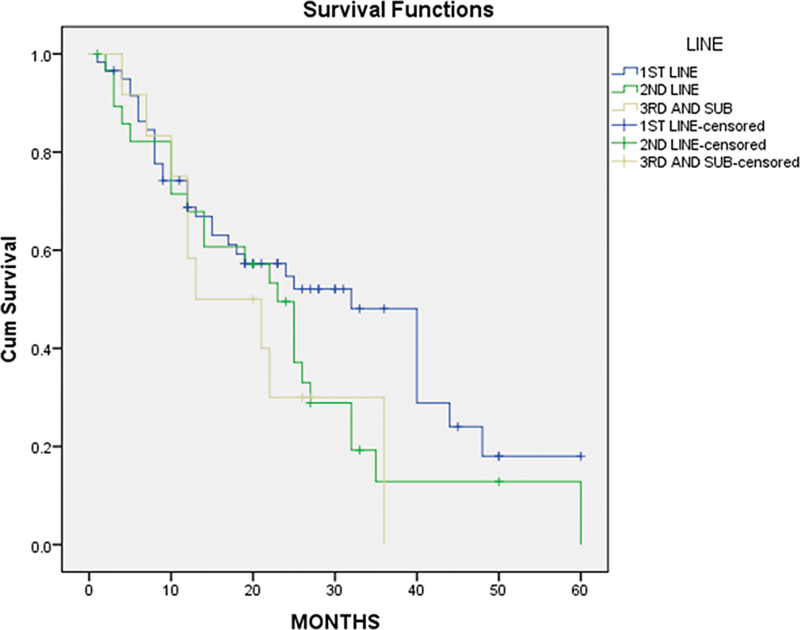
| Fig 2: Progression-free survival (PFS) based on different lines CDK4/6 used.
Discussion
The present study examined palbociclib's efficacy and safety in 100 HR-positive MBC patients with visceral metastases, finding a median PFS of 24 months. Bone metastasis had the longest PFS (27 months), followed by lung (25 months), liver (12 months), and brain (4 months). Common side effects included neutropenia (40%), anemia (35%), thrombocytopenia (15%), and hepatotoxicity (10%), with 3%-discontinuing treatment due to toxicity. Among these, two patients had prolonged neutropenia, and one patient had persistent liver enzyme elevation as well as generalized weakness due to the drug. Among the patients who progressed on palbociclib and underwent biopsy followed by molecular testing, 12%-started on PiK3 inhibitors (alpelisib), 46%-started on everolimus and exemestane, 32%-started on chemotherapy, and the remaining patients opted for best supportive care.
This is the only study evaluating the efficacy of palbociclib in different lines of metastatic hormone receptor-positive breast cancer.
In the landmark PALOMA-2 study,[10] which included 444 metastatic patients treated with palbociclib plus letrozole, the median PFS was 24.8 months, compared to 14.5 months with letrozole alone. Our study, with a median PFS of 24 months, despite 70%-of patients having prior ET, showed comparable outcomes. Notably, de novo metastatic disease in our cohort exhibited a median PFS of 40 months, significantly surpassing the PALOMA-2 trial. This divergence may be attributed to the selective use of palbociclib upfront in metastatic disease during the evolving era of CDK inhibitors. Common toxicities in the PALOMA-2 trial included neutropenia (66.4%), leukopenia (24.8%), anemia (5.4%), and fatigue (1.8%), with 1.8%-experiencing febrile neutropenia and 9.7%-discontinuing treatment, aligning with findings in our study.
In contrast, the initial PALOMA-3 trial[9] evaluated the efficacy of palbociclib plus fulvestrant in MBC patients who had progressed on previous ET, involving 345 patients with a median follow-up of 8.9 months. The median PFS was 9.5 months in those with visceral metastatic disease, whereas in patients with nonvisceral metastases, the median PFS was 16.6 months. In our study, PFS in visceral metastatic disease was 15 months compared to 27 months in skeletal metastasis, with acceptable toxicities, the most common being neutropenia, anemia, and thrombocytopenia. This disparity is due to the selection of patients with low visceral metastatic disease in our study during the initial days of CDK4/6i approval.
In the MONARCH 2 phase III RCT,[11] which evaluated abemaciclib plus fulvestrant in subgroup patients having visceral metastasis at presentation, results were more favorable than palbociclib plus ET with an hazard ratio of 0.48.
There are not many studies comparing CDK4/6 inhibitors head-on with chemotherapy drugs, especially in extensive visceral metastasis cases before CDK4/6 inhibitors were available. However, CDK4/6 inhibitors provide a chemotherapy-free regimen for HR-positive MBC without a visceral crisis.
In the RIGHT Choice trial, an open-label phase II study[12] conducted in 13 countries, a head-to-head comparison between a CDK4/6 inhibitor (ribociclib) plus ET and combination chemotherapy was evaluated. The study involved 112 patients receiving ribociclib plus ET, while in our current study, 100 patients received palbociclib plus ET. The percentage of patients with de novo metastatic disease was similar between the two studies (64.4%-in RIGHT Choice vs. 70%-in our study). However, there were notable differences in the patient populations: 67.6%-of patients in the RIGHT Choice trial had visceral metastasis compared to 44%- in our study, and 47%-of patients in the RIGHT Choice trial had a visceral crisis, while none in our study did. The median PFS in the ribociclib arm of the RIGHT Choice trial was 21.8 months, compared to 12.8 months in the chemotherapy arm. In our study, the median PFS in the palbociclib arm was 24 months. Regarding safety, grade 3 or 4 hematological toxicity occurred in 59.8%-of patients in the RIGHT Choice trial, whereas in our study, severe hematological toxicity was only 4%. Severe nonhematological toxicity was 3%-in the RIGHT Choice trial compared to 4%-in our study. There were five deaths in the ribociclib arm of the RIGHT Choice trial, compared to seven deaths in our study. The trial showed improved PFS, similar response rates, and lower rates of symptomatic adverse events with ribociclib plus ET compared to chemotherapy. In summary, the efficacy and safety results of our study are similar to those observed in the ribociclib arm of the RIGHT Choice trial.
In the FALCON study,[13] which compared fulvestrant with anastrozole, PFS was 22.3 versus 13.8 months in skeletal metastatic disease compared to 40 months in our study, with a comparable toxicity profile.
The KENDO randomized phase II trial[14] is the only study that compared the efficacy and safety of chemotherapy plus ET versus CDK4/6 inhibitors (CDK4/6i) plus ET in hormone receptor-positive (HR + )/HER2-negative MBC. The study found that CDK4/6i plus ET showed clinically meaningful improvements in PFS and OS compared to chemotherapy plus ET, although the difference was not statistically significant. Basal-like tumors under CDK4/6i plus ET had worse PFS and OS compared to other subtypes, while luminal A tumors performed worse with chemotherapy. The PAM50 intrinsic subtypes were found to have prognostic and predictive value, with luminal A associated with the best prognosis and basal-like with the worst prognosis. Genes and pathways involved in breast cancer cell survival and proliferation were associated with worse outcomes, while immune-related genes and signatures showed favorable survival trends, especially in the CDK4/6i arm. Tumor-infiltrating lymphocytes and the presence of tertiary lymphoid structures were associated with better outcomes in the CDK4/6i arm. CD24 was identified as a potential therapeutic target, and messenger ribonucleic acid-based CD19 and CXCL13 were found to be predictors of tertiary lymphoid structure presence. Overall, the results suggest that CDK4/6i plus ET is a viable treatment option for aggressive HR +/HER2-negative MBC instead of chemotherapy, and PAM50 intrinsic subtypes, genomic, and immunological features are promising biomarkers for personalized therapeutic choices.
Our study findings of palbociclib with ET in real-world data suggest that palbociclib plus ET, which is a widely used CDK4/6 inhibitor with ET, showed similar efficacy and safety comparable with RCTs in visceral metastatic disease without visceral crisis. This is the only study that evaluated the efficacy of palbociclib in multiple lines.
Conclusion
Based on real-world evidence, palbociclib demonstrates similar responses and better tolerance in visceral metastatic hormone positive breast cancer. However, further studies are required to identify additional predictive markers and factors related to CDK4/6 inhibitors resistance. Moreover, the efficacy of palbociclib inhibitors should be evaluated, particularly in patients with a high disease burden and extensive visceral metastatic disease.
Conflict of Interest
None declared.
Patient Consent
Informed patient consent was obtained to conduct this study.
References
- Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2020; 70 (04) 313-336
- Jonnada PK, Sushma C, Karyampudi M, Dharanikota A. Prevalence of molecular subtypes of breast cancer in India: a systematic review and meta-analysis. Indian J Surg Oncol 2021; 12 (Suppl. 01) 152-163
- Kumar RV, Panwar D, Amirtham U. et al. Estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2 status in breast cancer: a retrospective study of 5436 women from a regional cancer center in South India. South Asian J Cancer 2018; 7 (01) 7-10
- Pandit P, Patil R, Palwe V, Gandhe S, Patil R, Nagarkar R. Prevalence of molecular subtypes of breast cancer: a single institutional experience of 2062 patients. Eur J Breast Health 2019; 16 (01) 39-43
- Belachew EB, Sewasew DT. Corrigendum: molecular mechanisms of endocrine resistance in estrogen-receptor-positive breast cancer. Front Endocrinol (Lausanne) 2021; 12: 689705
- Cardoso F, Paluch-Shimon S, Senkus E. et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020; 31 (12) 1623-1649
- Gennari A, André F, Barrios CH. et al; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 2021; 32 (12) 1475-1495
- Finn RS, Crown JP, Lang I. et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16 (01) 25-35
- Cristofanilli M, Turner NC, Bondarenko I. et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016; 17 (04) 425-439
- Finn RS, Martin M, Rugo HS. et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375 (20) 1925-1936
- Sledge Jr GW, Toi M, Neven P. et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35 (25) 2875-2884
- Lu YS, Bin Mohd Mahidin EI, Azim H. et al. Final results of RIGHT Choice: Ribociclib plus endocrine therapy vs combination chemotherapy in premenopausal women with clinically aggressive HR+/HER2− advanced breast cancer. J Clin Oncol 2024; 42 (23) 2812-2821
- Robertson JFR, Bondarenko IM, Trishkina E. et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 2016; 388 (10063): 2997-3005
- Schettini F, Palleschi M, Mannozzi F. et al. CDK4/6-inhibitors versus chemotherapy in advanced HR+/HER2-negative breast cancer: results and correlative biomarker analyses of the KENDO randomized phase II trial. Oncologist 2024; 29 (05) e622-e634
Address for correspondence
Publication History
Article published online:
17 September 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Challenges in the Management of Lung Cancer: Real-World Experience from a Tertiary Center in South IndiaVishnu Gopal, South Asian Journal of Cancer, 2021
- Real-World Experience of Treating Young Adult Patients with Breast Cancer from a Single Center in Southern IndiaPriya Iyer, South Asian Journal of Cancer
- Clinicopathological Evaluation of Patients with Hormone Receptor–Positive HER2-Negative Metastatic Breast Cancer Progressing on Endocrine Treatment: A Real-Worl...S. Shanthala, et al., South Asian Journal of Cancer, 2023
- Real-World Outcome of Platinum-Based Chemotherapy in Advanced Breast Cancer (ABC): A Retrospective Study from a Tertiary Cancer Center in IndiaIndhuja Muthiah Vaikundaraja, Indian J Radiol Imaging, 2021
- Real-World Experience of AI-Assisted Endocytoscopy Using EndoBRAIN—An Observational Study from a Tertiary Care CenterAnudeep Katrevula, Journal of Digestive Endoscopy
- Safety and Current Status of the COVID-19 Vaccine among Patients with Breast cancer: A Cross-Sectional Study from China<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Exploring the potential of simple automation concepts for quantifying functional groups on nanomaterials with optical assays<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Expression Pattern of the Rice α-Amylase Genes Related with the Process of Floret Opening<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Afforestation increases microbial diversity in low-carbon soils<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- African Swine Fever Virus MGF110-5L-6L Induces Host Cell Translation Arrest and Stress Granule Formation by Activating the PERK/PKR-eIF2α Pathway<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">

| Fig 1 : Progression-free survival (PFS) based on metastatic sites.

| Fig 2: Progression-free survival (PFS) based on different lines CDK4/6 used.
References
- Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2020; 70 (04) 313-336
- Jonnada PK, Sushma C, Karyampudi M, Dharanikota A. Prevalence of molecular subtypes of breast cancer in India: a systematic review and meta-analysis. Indian J Surg Oncol 2021; 12 (Suppl. 01) 152-163
- Kumar RV, Panwar D, Amirtham U. et al. Estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2 status in breast cancer: a retrospective study of 5436 women from a regional cancer center in South India. South Asian J Cancer 2018; 7 (01) 7-10
- Pandit P, Patil R, Palwe V, Gandhe S, Patil R, Nagarkar R. Prevalence of molecular subtypes of breast cancer: a single institutional experience of 2062 patients. Eur J Breast Health 2019; 16 (01) 39-43
- Belachew EB, Sewasew DT. Corrigendum: molecular mechanisms of endocrine resistance in estrogen-receptor-positive breast cancer. Front Endocrinol (Lausanne) 2021; 12: 689705
- Cardoso F, Paluch-Shimon S, Senkus E. et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020; 31 (12) 1623-1649
- Gennari A, André F, Barrios CH. et al; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 2021; 32 (12) 1475-1495
- Finn RS, Crown JP, Lang I. et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16 (01) 25-35
- Cristofanilli M, Turner NC, Bondarenko I. et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016; 17 (04) 425-439
- Finn RS, Martin M, Rugo HS. et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375 (20) 1925-1936
- Sledge Jr GW, Toi M, Neven P. et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35 (25) 2875-2884
- Lu YS, Bin Mohd Mahidin EI, Azim H. et al. Final results of RIGHT Choice: Ribociclib plus endocrine therapy vs combination chemotherapy in premenopausal women with clinically aggressive HR+/HER2− advanced breast cancer. J Clin Oncol 2024; 42 (23) 2812-2821
- Robertson JFR, Bondarenko IM, Trishkina E. et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 2016; 388 (10063): 2997-3005
- Schettini F, Palleschi M, Mannozzi F. et al. CDK4/6-inhibitors versus chemotherapy in advanced HR+/HER2-negative breast cancer: results and correlative biomarker analyses of the KENDO randomized phase II trial. Oncologist 2024; 29 (05) e622-e634


 PDF
PDF  Views
Views  Share
Share

