Clinical and laboratory observation systemic lupus erythematosus and acute lymphocytic leukemia: An unusual case
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2011; 32(03): 154-156
DOI: DOI: 10.4103/0971-5851.92816
Abstract
The association of systemic lupus erythematosus (SLE) and myeloproliferative and lymphoproliferative malignancies is widely reported. There is scarce information available with regards to the association of SLE and malignancy in children. Usually, SLE precedes the onset of lymphoproliferative disease, but the neoplasia can occur earlier or even simultaneously. There are only five pediatric cases of SLE and associated acute lymphoblastic leukemia (ALL) reported in literature. All of these except one satisfied the revised American College of Rheumatology Criteria for SLE. Three of these cases developed SLE several years after successful treatment of ALL. While two cases reported had simultaneous onset of SLE and ALL, one of the cases in this two-patient series, did not fulfill ≥4 criteria for diagnosis of SLE. We present a case of a 3-year-old boy with SLE and coexistent ALL. To the best of our knowledge, only two such cases of simultaneous presentation of both these diseases are present in literature.
Publication History
Article published online:
06 August 2021
© 2011. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The association of systemic lupus erythematosus (SLE) and myeloproliferative and lymphoproliferative malignancies is widely reported. There is scarce information available with regards to the association of SLE and malignancy in children. Usually, SLE precedes the onset of lymphoproliferative disease, but the neoplasia can occur earlier or even simultaneously. There are only five pediatric cases of SLE and associated acute lymphoblastic leukemia (ALL) reported in literature. All of these except one satisfied the revised American College of Rheumatology Criteria for SLE. Three of these cases developed SLE several years after successful treatment of ALL. While two cases reported had simultaneous onset of SLE and ALL, one of the cases in this two-patient series, did not fulfill ≥4 criteria for diagnosis of SLE. We present a case of a 3-year-old boy with SLE and coexistent ALL. To the best of our knowledge, only two such cases of simultaneous presentation of both these diseases are present in literature.
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune inflammatory disorder characterized by a wide variety of associations and an unpredictable course. The association of SLE and myeloproliferative and lymphoproliferative malignancies is widely reported in the adult literature.[1–4] Most of the data show that the malignancy is detected following the diagnosis and treatment of SLE.[4] There is only scarce information available in regards to the association of SLE and malignancy in children. Usually, SLE precedes the onset of lymphoproliferative disease,[5–8] but the neoplasia can occur earlier[5] or even simultaneously.[9] Single cases have been described of SLE preceding acute lymphoblastic leukemia (ALL)[10] and of SLE appearing 5 years after complete remission of ALL induced by mercaptopurine.[11] We present a case of a 3-year-old boy with SLE and coexistent ALL. To the best of our knowledge, only two such cases of simultaneous presentation of both these diseases are present in literature.
CASE REPORT
A 3-year-old boy was presented with high-grade fever for 30 days. The fever was associated with intermittent episodes of swelling, pain and erythema of small joints of bilateral hands. There was no restriction of movement of joints. No other joints were involved. There was no history of rash, oral ulcer, loose stools, painful micturition, bleeding from any site, sore throat, pyoderma or breathlessness. The past history was not significant. The child had two elder siblings, both of whom were healthy.
On examination, the child had pallor but no icterus, cyanosis, clubbing, lymphadenopathy or edema. There was no apparent rash or oral ulcers. The joints were normal at the time of admission. Abdominal examination revealed liver 8 cm below costal margin and span of 12 cm. The spleen was palpable 3 cm in the splenic axis below costal margin. The cardiac and respiratory examination was normal.
The investigations revealed pancytopenia and the smear showed activated lymphocytes (Hb 7.1 g/dl, total leukocyte count=3500/μl, MCV=75.1 fl, reticulocyte count= 0.2%, platelet count=1.34×105/ μl), Peripheral smear showed normocytic normochromic RBCs with mild anisopoikilocytosis, fair number of microcytic hypochromic cells with few tear drop cells and occasional nucleated RBCs. WBC series showed the presence of 6% blasts and activated lymphocytes and platelet were reduced.
The biochemical investigations revealed normal kidney and liver function tests and serum electrolytes. The workup for tuberculosis, malaria, enteric was all negative. Ultrasound abdomen confirmed the findings of hepatomegaly (liver size=11.5 cm, coarsened echotexture) and splenomegaly (spleen size=7.5 cm and normal echotexture). Antinuclear antibody (ANA) and anti-double stranded DNA (anti-dsDNA) were both positive. The fundus examination revealed no evidence of uveitis.
Bone marrow aspirate was performed and smears revealed cellular marrow with 74% blasts. These blasts were 1.5-2 times the size of lymphocytes with scant agranular cytoplasm, nucleoli with irregular nuclear membrane, five chromatin and 0-1 nucleoli. There was suppression of other myeloid and erythroid series. Occasional megakaryocytes were seen. No hemoparasite was seen. The smears stained negative for myeloperoxidase suggestive of ALL.
DISCUSSION
The association of SLE and hematological malignancies is widely reported in the adult literature.[1–4] Most of the data show that the malignancy is detected after the diagnosis and treatment of SLE.[4] Usually, SLE precedes the onset of lymphoproliferative disease,[5–8] but the neoplasia can occur earlier[5] or simultaneously.[9] Our case is a 3-year-old boy with SLE and coexistent ALL. This patient described here serves to reiterate the importance of considering ALL in the differential diagnosis of SLE in children.
SLE was the foremost diagnostic consideration in our patients based on clinical manifestations (fever, arthritis and anemia) and serological abnormalities (ANA and anti-dsDNA positivity). Our patient satisfied four criteria for the diagnosis of SLE[12] i.e., arthritis, leukopenia, ANA positivity and antibody to dsDNA. Although many of the clinical and laboratory features in our patient could be accounted for on the basis of ALL, the major confounding feature was the presence of ANA and anti-dsDNA. The presence of ANA has been reported in patients with malignancies, but positive autoantibodies such as anti ds-DNA are not present. Anti ds-DNA is specific for SLE and favors the diagnosis in presence of compatible clinical features. In view of unexplained hepatosplenomegaly, bone marrow aspiration was done, which subsequently established the diagnosis of ALL. Therefore, we diagnosed our child to have coexistent SLE and ALL.
There are only five pediatric cases of SLE and associated ALL reported in literature. All of these except one case[13] satisfied the revised American College of Rheumatology Criteria for SLE.[12] Three of these cases[14–16] developed SLE several years after successful treatment of ALL. While two cases reported had simultaneous onset of SLE and ALL.[13] One of the cases in this two patient series, did not fulfill ≥4 criteria for diagnosis of SLE. The summary of cases is elaborated in Table 1.
Table 1
Summary of cases in literature of SLE associated with ALL
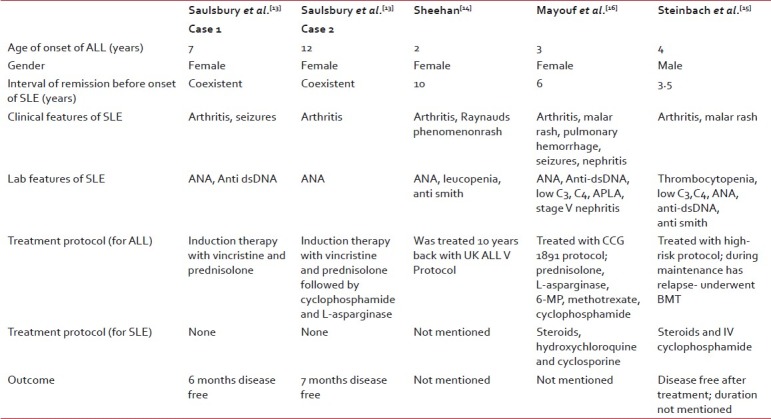
Various mechanisms have been postulated to explain the association between SLE and malignancies of the lymphoreticular system. The possibilities include a viral infection in a genetically susceptible host, facilitation of the neoplastic process by the autoimmune disorder, and suppression of immune surveillance by cytotoxic therapy. However, the increased susceptibility to malignancy of the lymphoid tissues in lupus appears to be independent of cytotoxic therapy.[2,9] Another possibility is that oncogene activation in the autoimmune diseases could initiate neoplasia. For instance, lymphocytes from patients with SLE exhibit increased expression of the proto-oncogenes c-myc and c-myb, transcripts of which are found in large amounts in lymphoid tumors.[17] SLE and ALL could simply be different expressions of the same immunological disorder as, although ALL is generally not associated with autoantibody formation, children with ALL have been found with positive test results for ANA in conjunction with clinical features of SLE.[13]
To conclude, findings in our patient serves to illustrate that ALL in children may mimic SLE. A bone marrow examination is therefore prudent in any child with a presumptive diagnosis of SLE or any other connective tissue disorders (CTD). Particularly if the clinical course is atypical, it is essential to rule out leukemia in any patient who present with clinical features of CTDs before starting corticosteroids.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.


 PDF
PDF  Views
Views  Share
Share

