Chronic myeloid leukemia treatment with Imatinib: An experience from a private tertiary care hospital
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(03): 182-185
DOI: DOI: 10.4103/0971-5851.123725
Abstract
The data presents 75 chronic myeloid leukemia patients diagnosed over a period 6 years i.e. from 2002 to 2008. The most common presentation was splenomegaly and 97% achieved complete hematological response at median duration of 4.3 weeks. The uniqueness of this study is follow-up with molecular response monitoring. Nearly, 30% patients achieved major molecular response (MMoR) by 12 months. 70% of patients achieved MMoR by median time of 60 months. Only 10% of the patient who achieved MMoR by 18 months had lost their responses subsequently.
Publication History
Article published online:
19 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The data presents 75 chronic myeloid leukemia patients diagnosed over a period 6 years i.e. from 2002 to 2008. The most common presentation was splenomegaly and 97% achieved complete hematological response at median duration of 4.3 weeks. The uniqueness of this study is follow-up with molecular response monitoring. Nearly, 30% patients achieved major molecular response (MMoR) by 12 months. 70% of patients achieved MMoR by median time of 60 months. Only 10% of the patient who achieved MMoR by 18 months had lost their responses subsequently.
INTRODUCTION
Chronic myeloid leukemia (CML) is the most common form of adult leukemia in India. Past decade has seen a major shift in the management and outcome of this once upon a time, uniformly fatal disease. Imatinib is presently the first line treatment of newly diagnosed CML in chronic phase, accelerated phase disease and even in blast crisis. Advances in translational research leading to better the drug development has seen the emergence of second generation tyrosine kinase inhibitors and src kinase inhibitors leading to successful treatment of Imatinib resistant cases. With these developments, CML is really becoming a chronic disease such as diabetes and hypertension. Imatinib became available to Indian patients in year 2002 and since then it has changed the scenario of CML in India. The present report describes our experience with this drug in patients of CML on follow-up.
PATIENTS AND METHODS
All adult patients diagnosed as CML at our unit during the period from 2002 to 2008, were treated with Imatinib. Patients, who were on regular follow-up and reported in the months of May and June 2010, were analyzed for side-effects and molecular response to treatment with Imatinib. All newly diagnosed patients were started on oral dose of 400 mg of Imatinib daily. The dose of Imatinib was increased if patients showed evidence of disease progression.
At diagnosis, all patients underwent complete physical examination, complete hemogram, and hepatic and renal function tests. After starting the treatment blood counts were performed weekly until patients achieved complete hematological response (CHR) and then monthly. All patients underwent quantitative assessment of BCR-ABL ratio by reverse-transcription polymerase chain reaction (RT-PCR) method at baseline and every 6 monthly. Bone marrow and cytogenetic studies were not performed routinely at follow-up as most of the patients were not keen for an invasive painful procedure and also because of logistics and technicalities associated with cytogenetic assessment.
Standard criteria for CHR, which included normalization of blood parameters and disappearance of clinical symptoms and signs including splenomegaly, major molecular response (MMoR) was defined as BCR-ABL/ABL ratio of less than 0.05% or 3 – log reduction from the baseline transcript values, whereas complete molecular response was defined as undetectable levels of BCR-ABL transcripts, were applied.
RESULTS
In our unit, 176 patients of CML were enrolled in Glivec International Patient Assistance Program (GiPAP) from 2002 till 2008. Of these 176 patients, 101 are still active on GiPAP registry and are on regular follow-up. The 75 patients presented to the out-patient department in the months of May and June 2010. The data of these 75 patients was analyzed for various parameters including toxicity profile and molecular responses.
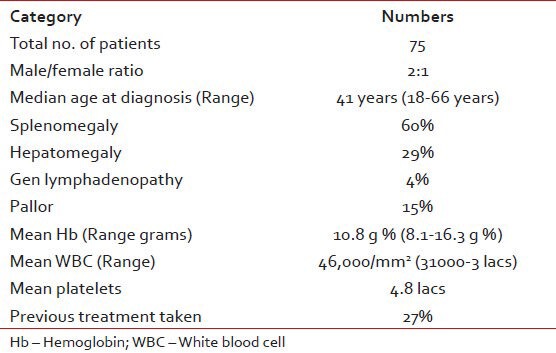
There were patients with median follow-up of months and patients have completed almost 10 years. The age varied between 18 and 66 years, median age was 61 years. Nearly 60% of patients presented with splenomegaly, 29% had hepatomegaly whereas only 4% had peripheral lymphadenopathy as shown in Table 1.
Table 1
Patient characteristics (demographics and presentation features) is as given below
Response evaluation
Hematologic response: Complete hematological response was seen in 97% of patients. The median time to attainment of CHR was 4.3 Weeks (Range from 2 weeks to 2½ months.)
Molecular response: In our analysis, 10% of patients had achieved MMoR by 6 months, 30% of the patients achieved MMoR by 12 months. By 18 months, 55% of patients had achieved MMoR and by 24 months 67% of patients had achieved MMoR as shown in figure figure11 and and2.2. Only 10% of patients, who had achieved MMoR, subsequently lost the molecular response.
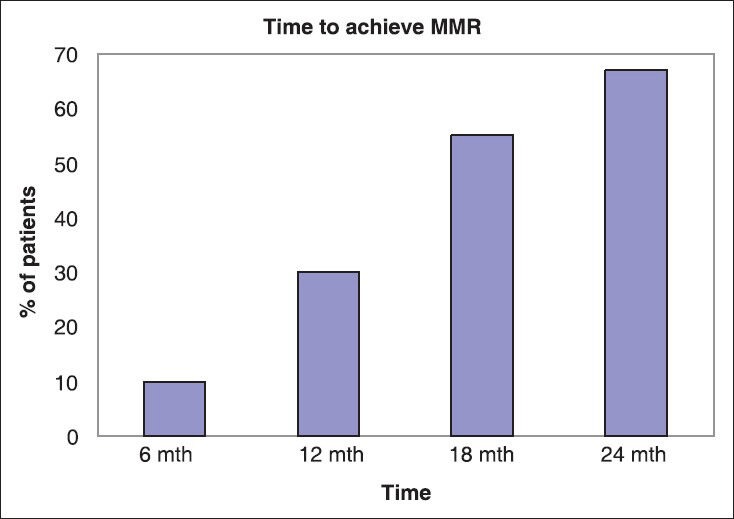
| Fig. 1 Showing Molecular response achieved by patient at different time intervals
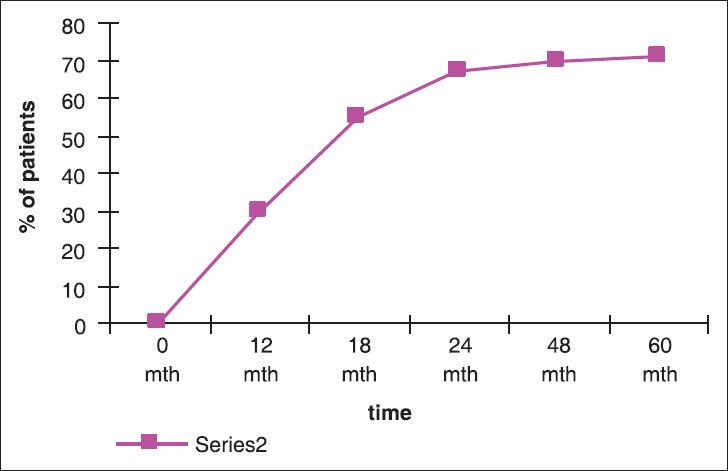
| Fig. 2 Showing trend in achievement of major molecular response as function of time
Dose escalation
Dose escalation was carried out in 10% of patients for loss of molecular response.
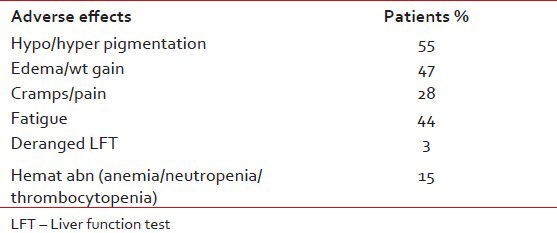
In the toxicities, as shown in Table 2, 55% of patients had skin pigmentation changes including malar rash, followed by complains of edema and or weight gain in 47% of patients. 44% of patients reported fatigue and 28% had crampy sensation or pains and aches. The Imatinib was well tolerated, 15% of patients had abnormalities of blood count parameters and only 3% had derangement of liver function test.
Table 2
Most common side-effects
DISCUSSION
The natural history of CML has seen a dramatic change with the introduction of Imatinib. In pre Imatinib era the average survival for patients used to be few years. Natural course of the disease used to pass through phases of chronic phase to accelerated phase to blast crisis and within a year or so of the blast crisis used to succumb to their disease. Now, we see patients maintained in chronic phase for a very long duration of time and accelerated phase and blast crisis have become uncommon in patients treated with Imatinib.
In our patient population, the median age was 41yrs (range 18-66yrs) which is concordance with other studies published from this subcontinent. The male female ratio in our study is 2:1, which may not be representative because of highly selective patient population studied. Other studies have also shown male preponderance.[1,2,3,4,5] Majority of patients presented with splenomegaly and the mean white blood cell count was 46,000 ranging from 36000-300000/cmm.
Hematological remission was obtained in 97% patients, which is in concurrence with the other studies.[1,3,5,6] The median time to CHR was 4.3 weeks (range from 2 weeks to 10 weeks).
Most of the studies reported so far from sub-continent have not reported molecular response assessment in their studies. The uniqueness of our study is follow-up with molecular response monitoring.
After starting on the treatment, the first to get corrected is hematologic parameters followed by cytogenetic and molecular criteria. Of which the molecular response assessment is the most sensitive and specific for CML, which is usually measured by quantitative RT-PCR, which can be easily performed on bone marrow or peripheral blood sample. Molecular monitoring using PCR is a sensitive assay that can measure disease burden even in patients who have achieved complete cytogenetic remission and thus may be able to identify patients at higher risk of resistance or relapse.
Wang et al. (2002)[7] in their study has shown that BCR-ABL/ABL ratio is an accurate surrogate of the contemporary marrow cytogenetic response in patients treated with Imatinib and in their subsequent studies have shown that early trends in BCR-ABL/ABL ratio enables prediction of cytogenetic response after 6 months of therapy. The other studies also suggest the same International Randomized Study of Interferon vs ST1571. (IRIS).
Nearly, 30% of our patients achieved MMoR s by 12 months and by 18 months 55% of our patients achieved MMoR s. Patients continued to achieve MMoR beyond 48 months and at median 60 months of follow-up we had a MMoR of about 70%. Only 10% of the patient who achieved MMoR by 18 months had lost their responses subsequently.
The study by Prasad et al.[8] showed that 37.5% patients achieved CMoR in first 6 months and remaining patients who did not achieve CMoR at 6 mo failed to do so even at 1 year also, which is in contrast to our observations that molecular response is an ongoing process, and there exists a sub set of patients who respond slowly, but go on to achieve molecular responses even later than 48 months period.
Another recently published large study from north India showed MMoR of 32%.[9]
It is accepted that molecular responses have prognostic significance. Patients who achieve early molecular responses are more likely to have durable cytogenetic responses along with better progression-free survival.
The molecular response assessment avoided the bone marrow examination procedure, which is a painful invasive test for which almost all of patients were reluctant to undergo periodically. Furthermore, the testing requires an intensive technical process, for which expertise and logistics might not be possible. However, cytogenetic testing may be reserved for special situations like increasing BCR-ABL transcripts, which may indicate loss of response and may help in detecting clonal evolution.
Thus monitoring molecular responses in CML may be a good parameter, especially in our country. Furthermore, by molecular response monitoring more timely decisions can be made regarding the investigative and management protocols besides predicting the therapeutic outcome.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Showing Molecular response achieved by patient at different time intervals

| Fig. 2 Showing trend in achievement of major molecular response as function of time


 PDF
PDF  Views
Views  Share
Share

