Childhood cancer in developing society: A roadmap of health care
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2011; 32(01): 30-33
DOI: DOI: 10.4103/0971-5851.81887
Abstract
Background: We assessed referral patterns of children with hematological malignancies (HM) in North India. Materials and Methods: The parents/guardians were interviewed at presentation, in the period between October 2001 and November 2002. Patient delay (symptom-contact), health system delay (contact-diagnosis), total delay (symptom-diagnosis), and number of contacts were compared between high- and standard-risk disease group. Results: Of the 79 children (55 boys; 69.6%) with HM, 47 (59.5%) had Acute Lymphoblastic Leukemia (ALL). Forty-four children had high-risk disease. The patient, system and total delay were a median of 2 days (with Interquartile range IQR of 1−6), 37 days (IQR 13−55), and 38 days (IQR 15−60) respectively. Majority of patients (64/79; 81%) went to private sector (non governmental health care providers) for health care. Number of contacts, which was the most significant, correlate with system delay. Conclusions: Sensitizing the private sector practitioners about cancer in symptomatic children (pallor, bleeding, fever) may be effective.
Publication History
Article published online:
16 August 2021
© 2011. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
We assessed referral patterns of children with hematological malignancies (HM) in North India.
Materials and Methods:
The parents/guardians were interviewed at presentation, in the period between October 2001 and November 2002. Patient delay (symptom-contact), health system delay (contact-diagnosis), total delay (symptom-diagnosis), and number of contacts were compared between high- and standard-risk disease group.
Results:
Of the 79 children (55 boys; 69.6%) with HM, 47 (59.5%) had Acute Lymphoblastic Leukemia (ALL). Forty-four children had high-risk disease. The patient, system and total delay were a median of 2 days (with Interquartile range IQR of 1–6), 37 days (IQR 13–55), and 38 days (IQR 15–60) respectively. Majority of patients (64/79; 81%) went to private sector (non governmental health care providers) for health care. Number of contacts, which was the most significant, correlate with system delay.
Conclusions:
Sensitizing the private sector practitioners about cancer in symptomatic children (pallor, bleeding, fever) may be effective.
INTRODUCTION
Estimated number of new cancers diagnosed in India every year is 700–900,000.[1] The geographic, socioeconomic, health system inequalities in cancer treatment, in children, have only now begun to be addressed.[2] Cancer remains the leading cause of death by disease in young individuals between 1 and 14 years of age.[3]
Advances in treatment in the past three decades have resulted in improved cure rates especially among children who are treated in a dedicated cancer care unit/center.[4,5] Early diagnosis is fundamental. It allows timely treatment and prevents unnecessary complications. Delays in health care with adverse events along the cancer care continuum may negatively impact prognosis. Long delays in diagnosis may adversely affect prognosis.[6–9] Previous studies have shown that time to diagnosis varies by cancer type, ranging from the shortest mean time to diagnosis of 2.5 weeks for renal tumors[10] to the longest time, which is 29.3 weeks for brain tumors.[11] It has been reported that the time for patients to report to a health professional is longer than the time needed for referral to a specialist.[12] Appropriate benchmarks for timely cancer care require a detailed understanding of the delays that may occur along the continuum of care.[13]
The Advanced Pediatric Centre, PGIMER, Chandigarh is a tertiary centre to which children from North and Western parts of the country are referred. Besides limitation in the existing health care systems, impact of delayed diagnosis on treatment initiation has not been quantified in our population.
Research question
What are the (probable) components for the delay in diagnosis of children (0–12 years) with hematological malignancies in developing countries like India?
MATERIALS AND METHODS
The parents/guardians of children with hematological malignancies (HM) were interviewed based on a predesigned performa at presentation in the period between the months of October 2001 and November 2002. Institutional ethics committee approval was obtained. Details available from previous prescriptions and referrals at hand were used in addition. Details of diagnosis (staging and risk categorization) were obtained from the case records maintained in the oncology clinic. HM included acute leukemia [lymphocytic (ALL), myeloid (AML), undifferentiated (AUL)] and non-Hodgkin's lymphoma (NHL). Risk stratification of disease category at presentation (into high- and standard-risk disease) was as per pre-existing universally accepted criteria. Patient delay (symptom-contact interval), health system delay (contact- diagnosis interval), total delay (symptom-diagnosis interval), and number of contacts were recorded in Excel spreadsheet and compared. Categorical variables were compared using Mann-Whitney U-test (bivariate) and Kruskal-Wallis (multivariate) rank test.
RESULTS
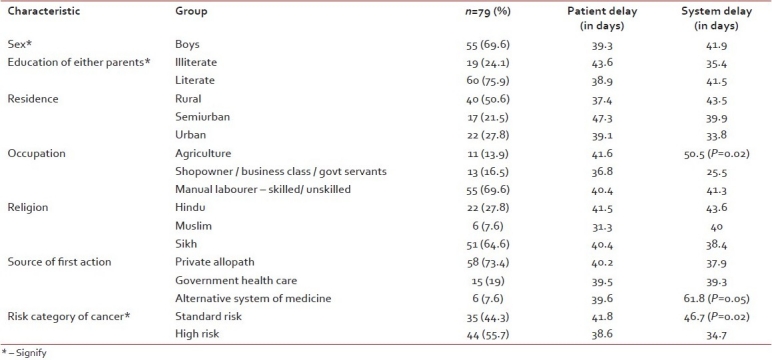
Of the 79 children (55 boys; 69.6%) with HM, the total number in high-risk category was 40 (50.6%), rest were of the standard risk group. The mean age was 5.9±3.2 years. The median patient delay of care seeking for children with high-risk HM was 2 (95% CI: 1.27, 3) days and for children with standard-risk HM was 3 (95% CI: 1, 4.22) days (P=0.42) [Table 1]. The median system delay of care providing for children with high-risk HM was 27 days and for children with standard risk HM was 40 days (P=0.19). System delay was significantly more for children of farmers, those who approached alternative health care systems, those with more number of different contacts with health system [Table 1]. There was no significant difference in patient delay, health system delay, and total delay among other groups based on religion, socioeconomic status, education status of parents, urban residence. The median income of parents of children with HM in the study was Rs. 2500 (Rs 500–20,000). The median number of health care visits by parents of children with cancer was 3 (range 1–10), before they were evaluated at the tertiary cancer care centre. None of the children had any medical insurance. After multiple logistic regressions and adjusting for factors, the number of contacts was the significant factor associated with longer health system delay for care seeker, in case of children with hematological malignancy.
Table 1
Access to care and patterns of care of children with hematological malignancy
DISCUSSION
We undertook a survey to determine the probable factors contributing to a delay in the presentation of children to our cancer care centre. We studied delays as intervals in the path of healthcare of childhood cancer patients without implying any value in terms of clinical acceptability [Figure 1].[13] We examined trends for the overall combination of delay times that can be considered as part of the referral pattern, that is the time elapsed from first medical contact by the patient until the diagnosis /onset of treatment. Diagnosis delay for all patients was approximately 1.5 month.
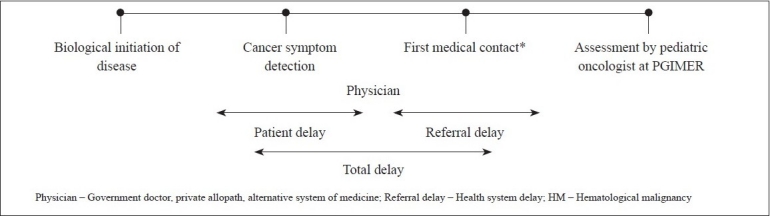
| Fig. 1 Components in pathway of care in childhood cancer (HM)
In our study, age, sex and interval from onset of symptom to contact with health system had no significant impact on the referral pattern. In a study of prognostic factors of ALL in India,[14] investigators have observed that age, sex, and phenotype of the disease had no significant impact on treatment outcome. As in our study, the baseline demography reflects a care seeking behavior preferring male infants and young boys.
The median patient delay for care seeking was only 2 days. Among young children, one expects that close parental observation of the child might help the recognition of symptoms and signs, whereas among older children and adolescents the recognition of signs and symptoms may be more often initiated by the patients themselves. Although Klein-Geltink et al.[12] reported that the patient delay is longer than the referral delay and that the patient delay is longer than the oncologist delay,[13] in the 79 children with HM studied, health system or referral delay (time to referral to specialist) was found to be the longest time segment responsible for driving the overall length of the delays [Figure 1]. Referral delay was influenced by number and type of contacts. Parents who were farmers often cited reasons of time of sowing, harvesting, marketing, which contributed to the significant delay in care seeking for their children after the first contact. Children with standard risk disease had a longer health system delay than high-risk disease. As reported by Saha et al.[10] also, tumor burden in young children with high risk disease may lead to faster progression of symptoms and therefore alert the health caregivers earlier. Often, physicians prescribed supportive care in the form of antipyretics, blood transfusionsm antibiotics, steroids with resultant delay in final diagnosis. Sensitizing the private sector practitioners about the possibility of hematological malignancy in symptomatic children (pallor, bleeding, fever) and the improved outcomes of care in a dedicated cancer care unit may be the most effective step in resource poor settings, for an early referral.
In India, the health system is designed to ensure that access to government medical services is provided to all citizens and paid for by public tax revenues without direct charges to the patient. Private health care system is also encouraged to supplement the public health delivery. People approach the private practitioners for health care first.
Limitations to this study: Firstly, its retrospective nature makes it difficult to ascertain the reliability and accuracy of the information collected. This may be particularly so for the reported initial onset of symptoms. The date information (primarily date of disease onset) was obtained from medical records and from patients or parents, which may have resulted in inaccurate recall. In conditions of poor referral systems, patients lose out on records in transit. However, we addressed it by the audits of prescription and referral slips and contacting referring physician whenever feasible at each centre within 24 hours of the patient's arrival at the hospital. Secondly, children with non-hematological malignancies were not included in this study, which would affect generalisability of our findings.
This study examines various delays from a regional perspective. Such studies across India may offer an opportunity to isolate the main time segment responsible for lengthening the cancer care pathway taken by children. This would enable evidence based decisions. Varying lengths of patient delay and referral delay, across settings, are the main contributors to delay in diagnosis. The information provided by a multicentric study,[15] may assist the implementation of intervention programs,[3] aimed at reducing delay where these can be most effective.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Components in pathway of care in childhood cancer (HM)


 PDF
PDF  Views
Views  Share
Share

