Chemotherapy-induced adverse drug reactions in oncology patients: A prospective observational survey
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(01): 42-46
DOI: DOI: 10.4103/0971-5851.177015
Abstract
Background: Chemotherapy, a multimodal approach to oncological treatment, involves highly complex regimens and hence accounts to high susceptibility toward adverse drug reactions (ADRs). The present study aims to determine the prevalence of adverse events in patients treated with chemotherapy. Materials and Methods: Spontaneous ADR report of patients on antineoplastic drugs received in the past 2 years (January 2011-January 2013) were studied. These reports were analyzed for various carcinomas under treatment, medications used, types of ADRs, organ system involvement, severity, causality assessment, and preventability. Results: Over a period of 2 years, a total 591 cases were received with an incidence of 58.6%. The prevalence of ADRs was more in female patients (73.6%) as compared to men. ADRs mostly occurred in the age group of 41-50 years (27.4%). Patients treated for breast carcinoma (39.1%) reported the highest incidence of ADRs. Cisplatin (19.6%) was found to be the most common offending drug. The most common ADR reported was nausea and vomiting (23%). Gastroenterology (40.1%) was the most affected system. About 50.2% of the ADRs required treatment and 12.9% ADRs were considered serious. Causality assessment revealed that 80% of the ADRs were possible. About 86.97% cases were found to be mild, and 51% were not preventable. Conclusion: The success of chemotherapy comes with the word of caution regarding toxicities of antineoplastic drugs. Pharmacovigilance of these drugs needs to be explored, and use of preventative measures needs to be enhanced in order to reduce the incidence and severity of ADRs.
Keywords
Adverse drug reaction - antineoplastic drugs - chemotherapy - oncology - pharmacovigilancePublication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Chemotherapy, a multimodal approach to oncological treatment, involves highly complex regimens and hence accounts to high susceptibility toward adverse drug reactions (ADRs). The present study aims to determine the prevalence of adverse events in patients treated with chemotherapy.
Materials and Methods:
Spontaneous ADR report of patients on antineoplastic drugs received in the past 2 years (January 2011-January 2013) were studied. These reports were analyzed for various carcinomas under treatment, medications used, types of ADRs, organ system involvement, severity, causality assessment, and preventability.
Results:
Over a period of 2 years, a total 591 cases were received with an incidence of 58.6%. The prevalence of ADRs was more in female patients (73.6%) as compared to men. ADRs mostly occurred in the age group of 41-50 years (27.4%). Patients treated for breast carcinoma (39.1%) reported the highest incidence of ADRs. Cisplatin (19.6%) was found to be the most common offending drug. The most common ADR reported was nausea and vomiting (23%). Gastroenterology (40.1%) was the most affected system. About 50.2% of the ADRs required treatment and 12.9% ADRs were considered serious. Causality assessment revealed that 80% of the ADRs were possible. About 86.97% cases were found to be mild, and 51% were not preventable.
Conclusion:
The success of chemotherapy comes with the word of caution regarding toxicities of antineoplastic drugs. Pharmacovigilance of these drugs needs to be explored, and use of preventative measures needs to be enhanced in order to reduce the incidence and severity of ADRs.
INTRODUCTION
Chemotherapy is employed as part of a multimodal approach to the treatment of many tumors.[1] Chemotherapy regimens are immensely complex, and cancer patients are a susceptible population with little tolerance.[2] The magnitude of adverse drug reactions (ADRs) endured by oncology patients is colossal making them almost synonymous with the treatment.[3] Epidemiological research performed in the Australia shows 11% of ADRs in Australian Hospitals were associated with antineoplastic drugs and immunosuppressive drugs with antineoplastic drugs being the most common agents responsible for medication-related hospitalizations.[4,5] Adding to this, Lau et al. obtained data through personal interviews with patients and comprehensive review of patients’ medical records and found that oncology patients have at least one ADR either on admission or during hospital stay, and over 40% have three or more ADRs, 88% of these ADRs were assessed to be predictable.[3]
A study done in Southern India also documents antineoplastic agents as the most common class of drugs causing the ADRs.[6] In addition to the earlier findings, a recent study analyzing global patterns of ADRs over a decade documented that high-income countries reported more ADRs for antineoplastic and immunomodulating agents.[7]
Besides their impact on human life, ADRs significantly influence health costs as well. Couffignal et al. estimated that the total cost to treat ADRs is 1.7% of the total budget of the hospital with a median cost of 8517 francs.[8] These results emphasize the high incidence and excess costs of ADRs related to anticancer chemotherapy.
Notwithstanding this, pharmacovigilance of these drugs is limited and characterized by a significant underreporting. Accordingly, it is imperative to measure the frequency and severity of ADRs experienced by oncology patients to broaden the knowledge on medicine safety and also to enable the development and implementation of intervention strategies to reduce the burden of ADRs.
Thus, the present study aims to determine the prevalence of adverse events, their nature and severity, as well as the treatments in the patients treated with cancer chemotherapy in a Tertiary Care Hospital.
MATERIALS AND METHODS
A prospective observational study conducted in the Department of Pharmacology, Lady Hardinge Medical College, New Delhi.
All the spontaneous adverse drug event reports due to cancer chemotherapeutic medications (complete with respect to all the required information), which were submitted to the adverse drug monitoring center at the Department of Pharmacology, Lady Hardinge Medical College, New Delhi, under the Pharmacovigilance Programme of India in the past 2 years (January 2011-January 2013) were included in the study. The study was approved by the Ethical Committee.
Patient related information (demographic details clinical and treatment data) were collected in a specially designed data collection form. Patients age, sex, diagnosis, suspected drugs causing ADRs, treatment details (dose, frequency, strength, date of starting and stopping), description of the event, onset and ablation of adverse event, information on challenge and dechallenge, duration of hospital stay, type of ADRs, system affected by the ADRs, outcome of the ADRs, and drugs used to manage the ADRs were analyzed.
Naranjo probability scale was used to evaluate the relationship between suspected ADR and the drug. The scale consists of a questionnaire which contains 10 questions with the options yes, no, and do not know, and the score was given for each option. The total score calculated from this questionnaire defines the category as >9: Definite, 5-8: Probable, and 1-4: Possible.[9]
The severity of the ADRs was determined using Hartwig Scale. According to this scale, ADRs were assessed as mild (level 1, 2), moderate (level 3, 4, 5), and severe (level 6, 7).[10]
Suspected ADRs were also categorized as serious and nonserious. Serious ADR was defined as any ADR which was fatal, life-threatening, permanently/significantly disabling, required initial hospitalization, or prolonged hospitalization, caused a congenital anomaly, required intervention to prevent permanent impairment or damage.[11]
The modified Schumock and Thornton criteria were used for determining the preventability of the ADR.[3]
The descriptive statistical analysis was performed with the SPSS software package version 19 Armonk, NY: IBM Corp.
RESULTS
During the study period of 2 years, 1008 patients received cancer chemotherapy. A total of 591 reports of ADRs were received with an incidence of 58.6%. The majority of females (73.6%) were found to have ADRs as compared to males (26.4%). The majority (27.4%) of the patients developing the ADRs were in the age group of 41-50 years [Table 1].
Table 1
Age distribution
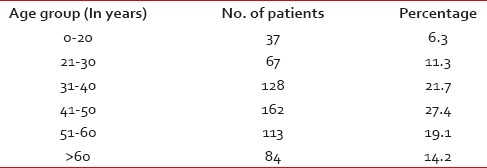
Patients received injection chlorpheniramine, injection dexamethasone, injection ranitidine, injection ondansetron, and tablet pantoprazole as premedications for different expected symptoms as per requirement of the patient.
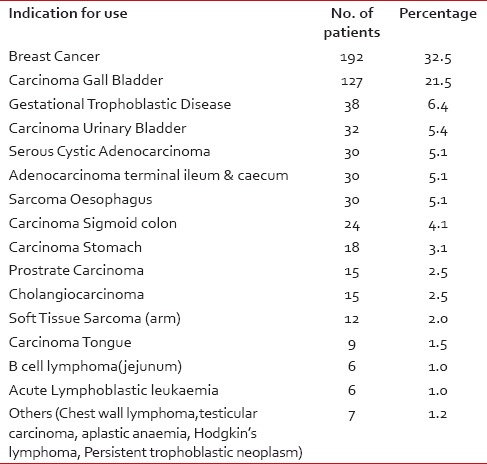
Among the patients, highest incidence of ADRs was seen in the patients undergoing treatment for breast carcinoma (39.1%), followed by carcinoma gallbladder (21.5%), gestational trophoblastic neoplasm (6.4%), and carcinoma urinary bladder (5.4%) [Table 2].
Table 2
Indication for use
The most affected system was gastroenterology (43.7%) followed by dermatology (24.9%) and hematology (23.2%) [Table 3].
Table 3
Types of ADRs
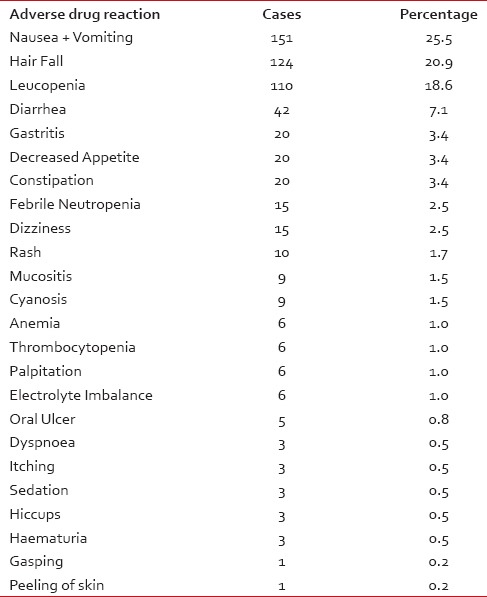
The frequently observed ADRs included nausea and vomiting (25.5%), hair fall (20.9%), leukopenia (18.6%) followed by diarrhea (7.1%), gastritis (3.4%) [Table 3].
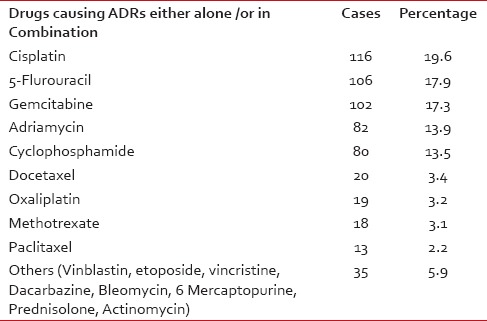
The common offending drugs causing ADRs either alone or in combination were cisplatin (19.6%), gemcitabine (17.3%) followed by 5-fluorouracil (5FU) (17.9%) [Table 4].
Table 4
Drugs causing ADRs either alone /or in Combination
Half (50.2%) of the ADRs required treatment. Injection ondansetron was the most common drug used for managing the ADRs followed by filgastrin, blood transfusion, dexamethasone, KCl, and ranitidine.
On causality assessment using Naranjo probability scale, 80% were possible and 20% ADRs were probable.
On severity assessment; according to the Hartwig Severity Scale, 514 ADRs (86.97%) were categorized as mild, 76 (12.8%) moderate, and 1 (0.17%) was found to be severe.
Regarding the outcomes attributed to ADRs, the majority of them were nonserious and only 76 (12.9%) ADRs were considered to be serious (leading to prolonged hospitalization). Among all the serious ADRs, one caused by cisplatin and 5FU was fatal. The patient developed gasping for which he was put on synchronized intermittent mandatory ventilation but despite this the patient died.
Over half (51%) of the ADRs were classified as not preventable, 42% probably preventable, and 7% definitely preventable.
DISCUSSION
The practice of cancer medicine has changed dramatically nowadays with treatment available for many previously fatal malignancies. Adjuvant chemotherapy has proven to extend life and prevent disease recurrence. Despite these therapeutic successes, many of the antineoplastic drugs possess narrow therapeutic index and a greater potential for causing adverse effects such as nausea/vomiting, neutropenia/anemia/pancytopenia, alopecia, constipation/diarrhea, and fatigue/tiredness.[1]
The demographic profile of the present study shows that majority of females (73.6%) were found to have ADRs as compared to males. This is consistent with other studies and the fact that women experience more adverse reactions to therapeutic drugs than men as a result of different pharmacokinetic and pharmacodynamic responses to drugs.[12,13,14]
On the contrary, there are studies where males were found to have more number of ADRs as compared to females.[15,16]
ADRs mostly occurred in the age group of 41-50 years, which is similar to that reported by other studies.[12,13] Some of the studies have found the most common age group to be between 50 and 70 years.[15,16] In general, the incidence of ADRs among older adults and elderly adults have been reported to be significantly higher than other age groups.[6]
In agreement to other studies, the highest incidence (39.1%) of ADRs was seen in patients undergoing treatment for breast carcinoma.[12,13]
In relation to the organ systems, most frequently involved in suspected ADRs, our results are consistent with previous studies,[13,16] where reactions affecting the gastrointestinal tract, were found to be among the most frequently observed events.
Contrary to this study, Mallik et al. reported hematological system to be the most frequently involved organ system, followed by the gastrointestinal tract.[15]
Similar to other studies, the most common ADRs found in this study were nausea and vomiting.[16] It was observed that majority of patients had received antiemetic as preventive therapy accordingly for them the dose of antiemetic was increased. This is consistent with findings of other studies where also the majority of the cases received increased doses of anti-emetic in order to manage ADR.[12,15]
Nevertheless with the use of 5-hydroxytryptamine 3 antagonist, the incidence of nausea and vomiting has significantly decreased though they have failed to prevent this completely. This indicates that current ADR prevention and management practices require attention.
Cisplatin was reported as the most common drug responsible for ADRs, which is consistent with that reported by other studies.[13,15,16,17] Whereas antimetabolites and alkylating agents were the most common drugs causing ADRs in Poddar et al. study.[12]
Cisplatin is a commonly used antineoplastic drug with major antitumor activity in a broad range of solid tumors, including nonsmall cell and small cell lung cancer, esophageal and gastric cancer, head and neck cancer, and genitourinary cancers, particularly testicular, ovarian, and bladder cancer. The major acute toxicity due to cisplatin includes nausea and vomiting. While nephrotoxicity, peripheral sensory neuropathy, ototoxicity, and nerve dysfunction forms the major delayed toxicities. Other well-documented ADRs of cisplatin includes mild to moderate myelosuppression (transient leukopenia, thrombocytopenia, anemia), hypomagnesemia, hypocalcemia, hypokalemia, hypophosphatemia, and anaphylactic-like reactions, characterized by facial edema, bronchoconstriction, tachycardia, and hypotension.[1]
The causality assessment revealed that most of the ADRs were “possible” followed by “probable” category. The majority of the ADRs reported in this study were mild (86.97%); nonetheless, they are a source of patient's agony. Less commonly, severe reactions were also observed.
Over half (51%) of the ADRs were classified as not preventable, which is in concordance with findings of other authors.[3]
Chemotherapy has dramatically changed the outcome of cancer patients. Despite this success, word of caution regarding toxicities of antineoplastic drugs deserves highlighting. It is vital to recognize these toxicities. Enhanced use of preventative measures and early detection of drug toxicity has the potential to contribute to reduce the severity of ADRs. Pharmacovigilance is unquestionably a public health interest; moreover enhancement in national reporting will contribute to better international reporting. Accordingly, a comprehensive and effective pharmacovigilance need to be put into place to reduce the burden of ADRs and thereby further improve the benefit: Harm ratio of the drugs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.


 PDF
PDF  Views
Views  Share
Share

