Cerebral aspergillus infection in pediatric acute lymphoblastic leukemia induction therapy
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(04): 236-238
DOI: DOI: 10.4103/0971-5851.107104
Abstract
Angioinvasive pulmonary infection from filamentous fungi is not an uncommon occurrence in immunocompromised patients like acute lymphoblastic leukemia (ALL). Rarely, these lesions can spread via the hematogenous route and involve multiple visceral organs. We report a case of a 14-year-old boy with ALL who developed angioinvasive pulmonary aspergillosis early in the course of induction therapy, which was followed by hematogenous dissemination and formation of multiple brain abscesses. The patient was treated with intravenous amphotericin B. There was no response to the therapy and the patient succumbed to disseminated infection. Postmortem lung biopsy confirmed angioinvasive pulmonary aspergillosis. Poor penetration of amphotericin B across the blood-brain barrier could be one of the contributory factors for poor response to antifungal therapy. We discuss the various antifungal agents with respect to their penetration in brain.
Keywords
Acute lymphoblastic leukemia - central nervous system aspergillosis - fungal brain abscess - fungal pneumoniaPublication History
Article published online:
20 July 2021
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Angioinvasive pulmonary infection from filamentous fungi is not an uncommon occurrence in immunocompromised patients like acute lymphoblastic leukemia (ALL). Rarely, these lesions can spread via the hematogenous route and involve multiple visceral organs. We report a case of a 14-year-old boy with ALL who developed angioinvasive pulmonary aspergillosis early in the course of induction therapy, which was followed by hematogenous dissemination and formation of multiple brain abscesses. The patient was treated with intravenous amphotericin B. There was no response to the therapy and the patient succumbed to disseminated infection. Postmortem lung biopsy confirmed angioinvasive pulmonary aspergillosis. Poor penetration of amphotericin B across the blood-brain barrier could be one of the contributory factors for poor response to antifungal therapy. We discuss the various antifungal agents with respect to their penetration in brain.
INTRODUCTION
Aspergillus is a ubiquitous branching septate filamentous fungus. It mainly gives rise to sinopulmonary infections, but hematogenous spread due to its angioinvasive nature is also known. Aspergillus infection of the brain may result from either contiguous spread of the infection from the paranasal sinuses[1] or via the hematogenous route. Many modern antifungal agents are active against Aspergillus, but the main factor in the treatment of central nervous system (CNS) aspergillosis is its penetration across the blood-brain barrier.
CASE REPORT
A 14-year-old boy with B-precursor acute lymphoblastic leukemia (ALL) presented to our emergency department with fever for 2 months, gum bleeding and breathlessness of 3 days duration. He was pale, dyspnic and hypotensive. In view of progressively worsening hypoxia, he was immediately put on assisted ventilation, inotropic support and intravenous antibiotics, including cephoperazone–sulbactam, amikacin and vancomycin. Baseline hemoglobin was 4.3 g/dL, white blood cell count 3900/mL and platelet count 14,000/mL, while the renal and liver function tests were normal. Chest radiograph showed bilateral lower zone consolidation. Antifungal prophylaxis was initiated with amphotericin B at a dose of 0.5 mg/kg on alternate days. Induction therapy for ALL was started with prednisolone and he was successfully extubated on Day 3. At this time, antileukemic therapy with steroids was continued and vincristine was administered. He developed hyperbilirubinemia of 7.8 mg/dL (direct bilirubin 6.2 mg/dL), aspartate transferase 46 IU/L, alanine transferase 121 IU/L and alkaline phosphatase 483 IU/L on Day 4, due to which daunorubicin and L-asparaginase were not administered. On Day 5 of induction therapy, he developed chest pain and aggravation of hypoxia. Chest radiograph showed bilateral multiple large nodular shadows predominantly in the lower lobes [Figure 1a]. High-resolution computerized tomography (CT) of the chest showed bilateral multiple nodules surrounded by halo, suggestive of angioinvasive pulmonary aspergillosis [Figure 1b]. Serum galactomannan ratio was positive at 3.0 (normal value < 0.50). He was switched to daily therapy with amphotericin B at 1.5 mg/kg. On Day 7 of induction therapy, he developed unconsciousness with anisocoria, papilledema and a fixed right-sided gaze. Contrast-enhanced CT scan of the head showed multiple well-defined, enhancing, hypodense nodules in both cerebral hemispheres with perilesional edema [Figure1c and d], suggestive of CNS aspergillosis. Steroids, a predominant risk factor for invasive fungal infection, were withheld temporarily; additionally, the patient was administered decongestive therapy with mannitol. His condition deteriorated and he succumbed on Day 9 of induction. Postmortem lung biopsy showed vasoinvasive aggregates of fungal hyphal forms with septations and branching suggestive of aspergillosis [Figure1e and f], although a culture was not done. In view of CT findings of chest, a positive galactomannan assay and lung biopsy, it was a proven case of pulmonary aspergillosis with hematogenous dissemination to the brain.
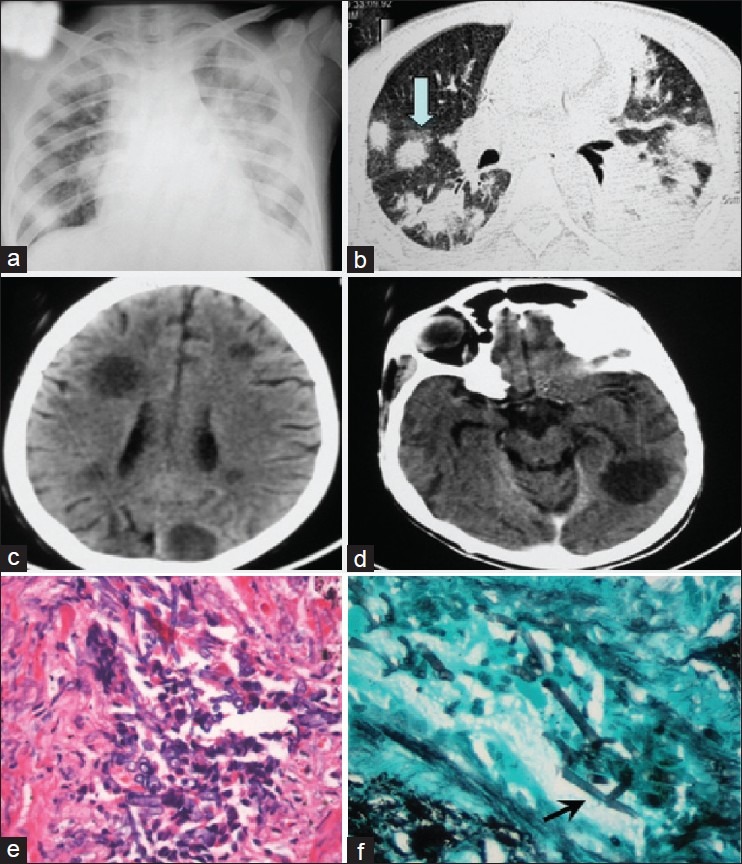
| Fig. 1 Chest radiograph showing multiple nodular consolidations in both lung fields (a); high-resolution computerized tomography (CT) chest showing multiple bilateral confluent nodular opacities in lungs, some of then showing surrounding ground glass halo (arrow) (b); contrast enhanced CT brain shows multiple rounded hypodence space-occupying lesions in both cerebral hemispheres (d and e); hematoxylin and eosin stain showing septate, dichotomously branched fungal hyphae (×40) (e); Gomori's methanamine silver stain highlighting the 45° branching hyphae (arrow) consistent with Aspergillus fungi (×40) (f)
DISCUSSION
Filamentous fungal infections are progressively increasing in patients of hematological malignancies. Both the compromised host defense and the neutropenia caused by cytotoxic therapy are attributable for the higher incidence of invasive mycoses in this subgroup of patients. Mycotic diseases in the brain are usually secondary to infections elsewhere in the body, usually the lungs, and in the vast majority of the cases, spread is via blood circulation. Alternatively, intracranial aspergillosis may result from direct extension of infections in the sinuses or bones.[2] In a large series of filamentous fungal infections, after lungs and paranasal sinuses, brain (9.4%) is the third most common site of fungal infection in patients of hematological malignancies, of which 8.7% cases were secondary infections[3] CNS mycosis can present as cerebral abscess/granuloma, arteritis, fungal embolization with ischemic infarction and mycotic aneurysm. In a series of 90 cases of CNS aspergillosis in children, the most common presentation was in the form of brain abscess, either single or multiple; leukemia was the most frequent underlying disease and Aspergillus fumigatus was isolated from 75.5% of the cases.[4] Treatment of CNS aspergillosis has been unsatisfactory, and mortality is reported to reach 100% in immunocompromised and 67% in immunocompetent hosts.[5,6] The main reason is that CNS is a sanctuary site and drug penetration is often inadequate. The penetration of drugs across the blood–brain barrier is mainly limited by their molecular size and physicochemical properties as well as drug interaction with transporter systems such as P-glycoprotein at the blood–brain barrier. Most antifungal agents are large molecules (>700 Da), which makes sufficient penetration into the cerebrospinal fluid and brain tissue unlikely, except for fluconazole and voriconazole.[7] Amphotericin B (either as deoxycholate or liposomal form), echinocandins like caspofungin and itraconazole poorly penetrate across the blood-brain barrier.[8] Voriconazole has better penetration in the CNS in humans with a cerebrospinal fluid to plasma ratio of 0.22 to 1. In addition, high voriconazole brain tissue levels (11.8 mcg/g and 58.5 mcg/g) were found at autopsy in two patients with pulmonary aspergillosis, indicating that voriconazole is enriched in brain tissue.[9] Further, voriconazole has also been shown to be effective in pediatric patients with cerebral aspergillosis.[10]
Our patient developed fungal pneumonia relatively early in the course of induction. Possibly, the late presentation to hospital resulted in Aspergillus infection progressing rapidly when corticosteroids were started. Further, voriconazole could not be administered because of the hyperbilirubinemia. Considering high mortality of disseminated filamentous fungal infection and poor outcome of treatment, we emphasize the need of early clinical suspicion of fungal infections and consideration of voriconazole-based antifungal therapy in patients with CNS aspergillosis based on the available literature of antifungal drug penetration in CNS.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Chest radiograph showing multiple nodular consolidations in both lung fields (a); high-resolution computerized tomography (CT) chest showing multiple bilateral confluent nodular opacities in lungs, some of then showing surrounding ground glass halo (arrow) (b); contrast enhanced CT brain shows multiple rounded hypodence space-occupying lesions in both cerebral hemispheres (d and e); hematoxylin and eosin stain showing septate, dichotomously branched fungal hyphae (×40) (e); Gomori's methanamine silver stain highlighting the 45° branching hyphae (arrow) consistent with Aspergillus fungi (×40) (f)


 PDF
PDF  Views
Views  Share
Share

