CDX2 Expression in Gastric Carcinoma: A Clinicopathological Study
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2018; 39(01): 52-57
DOI: DOI: 10.4103/ijmpo.ijmpo_49_17
Abstract
Background: Gastric cancer accounts for 7.8% of cancers worldwide and adenocarcinoma is the commonest histological type. Both gastric and intestinal phenotypic cell markers are expressed in gastric carcinomas. CDX2 is an intestinal transcription factor, which can be demonstrated in intestinal metaplasia and gastric carcinomas of the intestinal type. Unlike colorectal carcinomas, the role of CDX2 in gastric carcinomas as a prognostic variable is yet to be established. Ki-67 is a transcription factor expressed in the growth and synthetic phases of the cell cycle. Aims and Objectives: The aims of the study were to analyze CDX2 expression and Ki-67 labeling index in different histological types of gastric carcinomas and their relationship with the patients' clinicopathological parameters. Materials and Methods: A total of 50 gastric carcinoma cases were evaluated histologically and phenotypically, along with assessment of CDX2 expression and Ki-67 labeling index. Gastric carcinomas were grouped into intestinal and diffuse types, according to Lauren classification. A semiquantitative microscopic evaluation of CDX2 expression and Ki-67 labeling index was performed and correlated with the patients' clinicopathological parameters. Results: Increased CDX2 expression correlated with higher proportion of intestinal type gastric carcinomas and a lower proportion of lymph node metastasis, lymphovascular and perineural invasion. On the other hand, high Ki-67 labeling index was found in high grade tumors with lymphovascular invasion. Conclusions: The results of our study suggest that CDX2 might be a useful marker in predicting the prognosis of patients with gastric carcinoma. Accordingly, Ki-67 index seems to be useful in identifying a group of patients with aggressive tumors.
Keywords
Adenocarcinoma - CDX2 - gastric carcinoma - Ki-67
Publication History
23 June 2021 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:?Gastric cancer accounts for 7.8% of cancers worldwide and adenocarcinoma is the commonest histological type. Both gastric and intestinal phenotypic cell markers are expressed in gastric carcinomas. CDX2 is an intestinal transcription factor, which can be demonstrated in intestinal metaplasia and gastric carcinomas of the intestinal type. Unlike colorectal carcinomas, the role of CDX2 in gastric carcinomas as a prognostic variable is yet to be established. Ki-67 is a transcription factor expressed in the growth and synthetic phases of the cell cycle.?Aims and Objectives:?The aims of the study were to analyze CDX2 expression and Ki-67 labeling index in different histological types of gastric carcinomas and their relationship with the patients' clinicopathological parameters.?Materials and Methods:?A total of 50 gastric carcinoma cases were evaluated histologically and phenotypically, along with assessment of CDX2 expression and Ki-67 labeling index. Gastric carcinomas were grouped into intestinal and diffuse types, according to Lauren classification. A semiquantitative microscopic evaluation of CDX2 expression and Ki-67 labeling index was performed and correlated with the patients' clinicopathological parameters. Results: Increased CDX2 expression correlated with higher proportion of intestinal type gastric carcinomas and a lower proportion of lymph node metastasis, lymphovascular and perineural invasion. On the other hand, high Ki-67 labeling index was found in high grade tumors with lymphovascular invasion. Conclusions: The results of our study suggest that CDX2 might be a useful marker in predicting the prognosis of patients with gastric carcinoma. Accordingly, Ki-67 index seems to be useful in identifying a group of patients with aggressive tumors.
Keywords
Adenocarcinoma - CDX2 - gastric carcinoma - Ki-67
Introduction
Gastric carcinomas are malignant epithelial neoplasms of the stomach which accounts for 7.8% of cancers worldwide.[1] They represent a biologically and genetically heterogeneous group of tumors with multifactorial etiologies, both environmental and genetic.[1],[2] Still widely used, Lauren classification divides gastric cancer into two major histological types intestinal and diffuse on the basis of microscopic configuration and growth pattern.[3] Intestinal type carcinomas form glands with various degrees of differentiation while diffuse carcinomas consist of poorly cohesive cells with little or no gland formation.[4]
Precursor lesions of gastric carcinomas include gastritis and intestinal metaplasia. Both autoimmune gastritis and?Helicobacter pylori (H. pylori)-induced gastritis are associated with the development of intestinal metaplasia in the stomach and an increased risk of developing gastric carcinoma, mostly of intestinal type.[1]
CDX-2 is a caudal-related homeobox transcription factor whose expression in the adult is normally restricted to the intestinal epithelium. It is implicated in the development and maintenance of intestinal mucosa.[5] Highest levels of CDX-2 mRNA are found in the caecum and colon with lower levels in other tracts of the intestine but there is a lack of expression in the stomach.[6] Its role as a prognostic marker in colorectal carcinomas is well known whereas its role in the outcome of gastric carcinomas is not yet established. Gastric mucosa exhibiting intestinal metaplasia show CDX2 immunoreactivity in about 90% of cases, as compared to gastric carcinomas which show immunoreactivity in only 50% of the cases.[7],[8] Differentiated adenocarcinomas are characterized by a higher CDX2 expression than undifferentiated tumors, with a stronger reactivity in the intestinal phenotypes. Recent studies report an inverse correlation between CDX2 expression and the depth of invasion as well as lymph node metastasis.[8],[9] Ki-67 is a nuclear proliferation-associated antigen expressed in the growth and synthetic phases of the cell cycle, thereby providing a direct measure of the growth fraction of the tissue.[10]
In the present study, we analyze the CDX2 expression and Ki-67 labeling index in different histological types of gastric carcinomas along with the correlation of the staining results with the patients' clinicopathological parameters.
Materials and Methods
Case selection and tissue samples
The present study was done over a period of one and half years between April 2013 and October 2014. It is a hospital-based observational study with cross-sectional type of study design. The study comprised of fifty patients who underwent total or partial gastrectomy between 2012 and 2014. The demographic details and clinical history of the patients were collected. Patients who died within 4 weeks after the surgical intervention were excluded from the study.
Tissue preparation
Gross examination of the surgically removed specimen was done, followed by grossing and block preparation for routine hematoxylin and eosin staining. Two additional sets of slides were prepared from each block for CDX2 and Ki-67 immunostaining, respectively. Histopathological findings included histological type and differentiation, depth of invasion, lymph node status, and lymphovascular invasion (LVI) and perineural invasion (PNI).
Immunohistochemistry
For immunohistochemistry, antigen retrieval was done with citrate buffer by microwaving (800 watt, 2 cycles of 5 min each followed by 600 watt, 1 cycle for 5 min). The slides were cooled to room temperature for 20 min. The slides are then washed in Tris-buffer, thrice for 5 min each. Endogenous peroxide activity was blocked by 0.3%. Hydrogen peroxidase in methanol. After washing once with water and twice with Tris-buffered saline (pH 7.6), incubation with primary monoclonal antibodies directed against CDX2 (Cell Marque, Rocklin, CA, USA) and Ki-67 (Dako, Glostrop, Denmark) was done for 60 min. The slides were then washed with Tris-buffer twice for 5 min each and the secondary or link antibody was applied. Horseradish peroxide polymer was added and incubated for 30 min at room temperature. Finally, the chromogen diaminobenzidine (DAKO) was added and incubated for 10 min followed by washing in tap water for 3 min. Counterstaining with hematoxylin and mounting concluded the immunohistochemical staining procedure.
For CDX2, a semiquantitative microscopic evaluation was performed by two pathologists independently. Nuclear staining was scored according to the percentage of positive tumor cells as follows Score 0: 0%?5% positive tumor cells; Score 1: >5%?35% positive tumor cells; Score 2: >35%?65% positive tumor cells; and Score 3: >65% positive tumor cells. Cases with score 0 were regarded as negative.
For Ki-67, a positive immunoreaction was considered for any degree of nuclear staining, and the cases were classified into two categories ? Low Ki-67 index (<20>20% staining).
Statistical analysis
Statistical analysis was done in Excel spread sheet. Pearson's Chi-square test and Fisher exact test was done to study the correlation of different parameters. A P < 0>
Results
The study comprised of a total of 50 cases of gastric carcinomas, which included 31 males and 19 females with a mean age of 51.2 years (ranging from 25 to 70 years). The majority of patients had a nonvegetarian dietary habit (41 cases, 82.0%). Among the cases, 33 cases (66.0%) were smokers.
Histologically, the gastric carcinomas were classified into intestinal and diffuse types, according to the Lauren classification. The intestinal type carcinomas were further graded histologically into well, moderate, and poorly differentiated forms based on the percentage of glandular differentiation. Twenty-two cases were of diffuse phenotype. Among the 28 cases of intestinal type, most of the cases were moderately differentiated (14 cases, 50.0%). In total, 31 cases had an invasion up to the serosal layer, out of which most were of the diffuse phenotype (18 cases, 58.06%). LVI and PNIs were present in 17 cases of diffuse type (77.27%). Among the intestinal type tumors, 11 cases (39.28%) showed LVI and 8 cases (28.57%) showed PNI. Overall, 27 cases showed lymph node involvement among which 17 cases (34.0%) were of diffuse type and 10 cases of intestinal type (20.0%).
The clinicopathological parameters have been summarized in [Table 1].
|
Parameter |
Number of cases, n (%) |
|||
|---|---|---|---|---|
|
Demographic parameters |
||||
|
LVI ? Lymphovascular invasion; PNI ? Perineural invasion |
||||
|
Age (years) |
||||
|
21-30 |
3 (06.0) |
|||
|
31-40 |
6 (12.0) |
|||
|
41-50 |
16 (32.0) |
|||
|
51-60 |
17 (34.0) |
|||
|
61-70 |
8 (16.0) |
|||
|
Mean age Sex |
52.16 |
|||
|
Males |
31 (62.0) |
|||
|
Females |
19 (38.0) |
|||
|
Dietary habits |
||||
|
Purely vegetarian |
9 (18.0) |
|||
|
Nonvegetarian |
||||
|
Smoking |
41 (82.0) |
|||
|
Smokers |
33 (66.0) |
|||
|
Nonsmokers |
17 (34.0) |
|||
|
Histopathological parameters |
||||
|
Histologic type and grade |
||||
|
Diffuse |
22 (44.0) |
|||
|
Intestinal |
28 (56.0) |
|||
|
Well |
||||
|
differentiated |
6 (12.0) |
|||
|
Moderately |
||||
|
differentiated |
14 (28.0) |
|||
|
Poorly |
||||
|
differentiated |
8 (16.0) |
|||
|
Diffuse |
Intestinal |
|||
|
Depth of invasion |
||||
|
Lamina propria |
1 (2.0) |
4 (8.0) |
||
|
Submucosa |
1 (2.0) |
3 (6.0) |
||
|
Muscularis propria |
3 (6.0) |
8 (16.0) |
||
|
Serosa |
17 (34.0) |
13 (26.0) |
||
|
Present |
Absent |
Present |
Absent |
|
|
LVI 17 |
(34.0) |
5 (10) |
11 (22.0) |
17 (34.0) |
|
PNI 17 |
(34.0) |
5 (10.0) |
8 (16.0) |
20 (40.0) |
|
Involved |
Uninvolved |
Involved |
Uninvolved |
|
|
Lymph node status |
17 (34.0) |
5 (10.0) |
10 (20.0) |
18 (36.0) |
|
Histological type |
Total (%) |
P |
||
|---|---|---|---|---|
|
n(%) |
n(%) |
|||
|
CDX2 ? Caudal type homeobox protein 2 |
||||
|
CDX2 score |
||||
|
0 |
21 (42.0) |
0 |
42.0 |
<0> |
|
1 |
1 (02.0) |
1 (2.0) |
4.0 |
|
|
2 |
0 |
12 (24.0) |
24.0 |
|
|
3 |
0 |
15 (30.0) |
30.0 |
|
|
Ki-67 proliferation index |
||||
|
Low Ki-67 (<20> |
3 (6.0) |
13 (26.0) |
32.0 |
0.0168 |
|
High Ki-67 (>20%) |
19 (38.0) |
15 (30.0) |
68.0 |
|
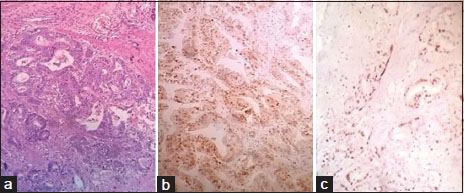
|?Figure.1(a) Intestinal type carcinomas with well-formed glands, (H and E, ?40). (b) Strong CDX2 [removed]3+) in intestinal type carcinoma. (c) Low Ki-67 index (<20>
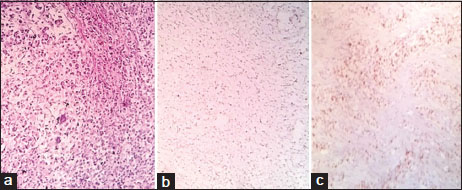
|?Figure.2(a) Diffuse type carcinomas with signet ring morphology, (H and E, ?40). (b) Weak CDX2 [removed]1+) in diffuse type carcinoma. (c) High Ki-67 index (>20%) in diffuse type carcinoma
Out of the 27 cases with nodal metastasis, a high Ki-67 index was found in 26 cases (96.3%), which was statistically highly significant (P?< 0 class="i" xss=removed>P?= 0.0051) and PNI (21 cases, 84.0%;?P?= 0.0322).
The correlation of different histopathological parameters with the CDX2 immunoreactivity and Ki-67 proliferation index is summarized in [Table 3].
|
CDX2 immunoreactivity |
Ki-67 proliferation index |
|||||||
|---|---|---|---|---|---|---|---|---|
|
0 (n=21) |
1 (n=2) |
2 (n=12) |
3 (n=15) |
P |
Low (<20 class="i">n=16) |
High (>20%) (n=34) |
P |
|
|
LVI ? Lymphovascular invasion; PNI ? Perineural invasion; CDX2 ? Caudal type homeobox protein 2 |
||||||||
|
Depth of invasion |
||||||||
|
Lamina propria |
1 |
1 |
0 |
3 |
>0.05 |
4 |
1 |
<0> |
|
Submucosa |
1 |
1 |
0 |
2 |
3 |
1 |
||
|
Muscularis propria |
4 |
0 |
5 |
2 |
8 |
3 |
||
|
Serosa |
15 |
0 |
7 |
8 |
3 |
27 |
||
|
LVI |
||||||||
|
Present |
16 |
1 |
7 |
4 |
0.0195 |
4 |
24 |
0.0051 |
|
Absent |
5 |
1 |
5 |
11 |
12 |
10 |
||
|
PNI |
||||||||
|
Present |
15 |
2 |
5 |
3 |
0.0059 |
4 |
21 |
0.0322 |
|
Absent |
6 |
0 |
7 |
12 |
12 |
13 |
||
|
Lymph node status |
||||||||
|
Uninvolved |
5 |
1 |
6 |
11 |
0.0304 |
15 |
8 |
<0> |
|
Involved |
16 |
1 |
6 |
4 |
1 |
26 |
||
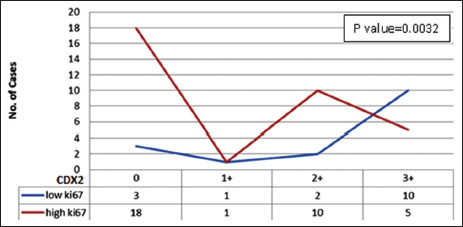
|?Figure.3Correlation between CDX2 and Ki-67
Discussion
Gastric carcinoma is one of the most common causes of cancer-related death worldwide. Newer parameters and markers are being used more frequently to detect and prognosticate these tumors. Gastric carcinomas are rare in persons below 30 years, and its incidence increases progressively with age.
CDX2 as a prognostic marker in colorectal cancers is well documented. However, its role as a prognostic marker in other carcinomas including gastric carcinoma is yet to be established.
Yu et al. in their study found that the percentage of female cases gradually decreased with age whereas that of the male cases were reverse.[11] Janssen et al. in their study found no difference in the rates of diffuse gastric carcinoma between the sexes. However, the rate of male patients with intestinal type carcinomas was more than twice as high as that of women.[12] Saha et al. in their study found a median age of 55 years with male:female sex ratio of 2.7:1.[13] In the present study, the mean age was 51.16 years with a male:female ratio of 1.63:1.
Environmental factors are strongly associated with gastric carcinomas. Besides H. pylori infection, tobacco smoking and dietary factors are the most important risk factors. Machida-Montani et al. in their study found a strong association of H. pylori infection and smoking with noncardiac gastric carcinomas.[14] Lee and Derakhshan in their study also found smoking and nonvegetarian food habit and excess salt intake to be strong independent risk factors of gastric cancers.[15] In our study too, about 66.0% of cases were smokers and 82.0% of cases had a nonvegetarian dietary habit.
Henson et al.[16] and Wu et al.[17] in their study of gastric carcinomas found a progressive increase in the diffuse type of gastric carcinoma, with respect to age. However, Saha et al.[13] and Lundeg?rdh et al.[18] in their study postulated the intestinal subtype being significantly more common among elderly people than in the younger age groups. In our study too, the majority of the cases were intestinal among which most were moderately differentiated. Cambruzzi et al. in their study found a predominance of lesions classified as T3 and N1.[19] This finding is similar to our study where 82?ses were advanced gastric cancer, of which T3 lesions predominated.
According to Liu et al., the presence of LVI is an important prognostic factor for gastric cancers that show no lymph node metastasis, with survival rate being lower in cases where lymphatic invasion was detected.[20] In this study, we found both LVI and PNI were present in 77.27% of diffuse phenotype carcinomas whereas among the intestinal types, LVI and PNI were observed in 39.28% and 28.57% of the cases, respectively. Overall, 54?ses show lymph node involvement of which 34?ses were of diffuse type and 20% were intestinal. These findings corroborated with the findings of Secondo Folli et al.[21] However, the findings were in sharp contrast to the findings of Cambruzzi et al., who found no significant relationship with histological grade and Lauren's histological type.[19]
CDX2 represents a transcription factor for various intestinal genes and thus an important regulator of intestinal differentiation which could be identified in intestinal metaplasia and gastric carcinomas. Ha Kim et al.[22] in his study, found that increased CDX2 expression correlated with a higher proportion of intestinal-type cancers and a lower proportion of PNI and lymph node metastasis. Advanced gastric cancers showed decreased CDX2 expression compared with early gastric cancer. There was no significant correlation between CDX2 expression and LVI. Similar findings were also documented by Roessler et al.[23] and Fan et al.[24] In our study too, all intestinal type cases were CDX2 positive of which 15 cases showed strong positivity (3+). Only one case of diffuse type was CDX2 positive. A positive correlation has been observed between strong CDX2 expression and intestinal differentiation (P < 0 xss=removed>
Lazar et al. observed a close correlation between the degree of tumor differentiation and the Ki-67 score.[10] However, the results of the study did not reveal any correlation between the Lauren's Classification of gastric carcinomas, the LVI, the depth of tumor invasion, the TNM stage and the Ki-67 score (P > 0.05). Ramires et al. in their study also that Ki67 LI of diffuse carcinomas were not significantly different from that of intestinal carcinomas. Ki67 LI was significantly higher (P = 0.006) in superficial than in deep areas regardless of histological tumor type. No significant relationship was observed between Ki-67 LI and wall invasion, lymph node metastasis, vascular invasion or ploidy.[25] In the study, all cases were Ki-67 positive, among which 68?ses had high Ki-67 index, of which majority were of diffuse type. The correlation between diffuse subtype and high Ki-67 index was statistically significant (P = 0.0168). LVI and PNI associated with high Ki-67 index were present in 70.6% and 61.8?ses respectively whereas only 25% with low Ki-67 index had both LVI and PNI. Thus, high Ki-67 index strongly correlated with the presence of LVI and PNI (P = 0.0051). About 85.3?ses with high Ki-67 index had lymph node involvement, which was statistically significant (P < 0>
Conclusions
CDX2 is an important marker for intestinal metaplasia and intestinal type of gastric adenocarcinomas. Higher grades of CDX2 positivity are associated with early gastric cancers and lower rates of lymph nodal metastasis. Hence, these results suggest that CDX2 might be a useful marker in predicting the prognosis of patients with gastric adenocarcinomas. Ki-67 LI on the other hand, is helpful in differentiating between the different histological grades and is a useful prognostic marker in identifying the group of patients with aggressive tumors. There is also an inverse relation between the degree of CDX2 expression and Ki-67 positivity.
Conflict of Interest
There are no conflicts of interest.
References
- osman FT, Carneiro F, Hruban RH, Theise ND.?WHO Classification of Tumors of the Digestive System. Lyon: IARC 2010; 4: 48-58
- arkin DM.?International variation. Oncogene 2014; 23: 6329-34
- erlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM.?Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893-917
- auren P, Sarin YK.?The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31-49
- uh E, Chen L, Taylor J, Traber PG.?A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol Cell Biol 1994; 14: 7340-51
- izoshita T, Inada K, Tsukamoto T, Kodera Y, Yamamura Y, Hirai T.?et al.?Expression of CDX1 and CDX2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa with special emphasis on participation in intestinal metaplasia of the human stomach. Gastric Cancer 2001; 294: 470-9
- lmeida R, Silva E, Santos-Silva F, Silberg DG, Wang J, De Bol?sC.?et al.?Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol 2003; 199: 36-40
- atoh K, Mutoh H, Eda A, Yanaka I, Osawa H, Honda S.?et al.?Aberrant expression of CDX2 in the gastric mucosa with and without intestinal metaplasia: Effect of eradication of Helicobacter pylori. Helicobacter 2002; 7: 192-8
- eno H, Oshima M, Taniguchi MA, Usami K, Ishikawa TO, Chiba T.?et al.?CDX2 expression in the stomach with intestinal metaplasia and intestinal-type cancer: Prognostic implications. Int J Oncol 2002; 21: 769-74
- Lazar D, Taban S, Sporea I, Dema A, Cornianu M, Lazar E.?et al.?Ki-67 expression in gastric cancer. Results from a prospective study with long-term follow-up. Rom J Morphol Embryol 2010; 51: 655-61
- Yu J, He Y, Guo Z.?Age trend of the male to female sex ratio in surgical gastric cancer patients at a single institution. World J Surg Oncol 2014; 12: 269
- Janssen CWJr, Maartmann-Moe H, Lie RT, Matre R.?Age and sex distribution of intestinal type and diffuse gastric carcinoma. APMIS 1991; 99: 78-82
- Saha AK, Maitra S, Hazra SC.?Epidemiology of gastric cancer in the gangetic areas of West Bengal. ISRN Gastroenterol 2013; 2013: 823483
- trong>?Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y.?et al.?Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer 2014; 7: 46-53
- Lee YY, Derakhshan MH.?Environmental and lifestyle risk factors of gastric cancer. Arch Iran Med 2013; 16: 358-65
- Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J.?Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: Increase in the signet ring cell type. Arch Pathol Lab Med 2004; 128: 765-70
- Wu H, Rusiecki JA, Zhu K, Potter J, Devesa SS.?Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev 2009; 1945: 52
- Lundeg?rdh G, Lindgren A, Rohul A, Nyr?n O, Hansson LE, Bergstr?m R.?et al?Intestinal and diffuse types of gastric cancer: Secular trends in Sweden since 1951. Br J Cancer 1991; 64: 1182-6
- Cambruzzi E, Azeredo AM, Kronhart A, Foltz KM, Zettler CG, P?gas KL.?The presence of metastases in regional lymph nodes is associated with tumor size and depth of invasion in sporadic gastric adenocarcinoma. Arq Bras Cir Dig 2014; 27: 18-21
- Liu Y, Chen XH, Meng XH, Liu CF, Zhao LL, Han JW.?et al.?Multivariate prognostic study on node-positive gastric cancer: Is tumor size a prognostic indicator?. Hepatogastroenterology 2012; 59: 623-6
- Folli S, Morgagni P, Roviello F, De ManzoniG, Marrelli D, Saragoni l.?et al.?Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol 2001; 31: 495-9
- Ha KimG, Am SongG, Youn ParkD, Han LeeS, Hyun LeeD, Oh KimT.?et al.?CDX2 expression is increased in gastric cancers with less invasiveness and intestinal mucin phenotype. Scand J Gastroenterol 2006; 41: 880-6
- Roessler K, M?nig SP, Schneider PM, Hanisch FG, Landsberg S, Thiele J.?et al?Co-expression of CDX2 and MUC2 in gastric carcinomas: Correlations with clinico-pathological parameters and prognosis. World J Gastroenterol 2005; 11: 3182-8
- Fan Z, Li J, Dong B, Huang X.?Expression of Cdx2 and hepatocyte antigen in gastric carcinoma: Correlation with histologic type and implications for prognosis. Clin Cancer Res 2005; 11: 6162-70
- Ramires M, David L, Leit?o D, Seixas M, Sansonetty F, Sobrinho-Sim?e M.?Ki67 labelling index in gastric carcinomas. An immunohistochemical study using double staining for the evaluation of the proliferative activity of diffuse-type carcinomas. J Pathol 1997; 182: 62-7
Address for correspondence
Publication History
23 June 2021 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Figure.1(a) Intestinal type carcinomas with well-formed glands, (H and E, ?40). (b) Strong CDX2 [removed]3+) in intestinal type carcinoma. (c) Low Ki-67 index (<20>

|?Figure.2(a) Diffuse type carcinomas with signet ring morphology, (H and E, ?40). (b) Weak CDX2 [removed]1+) in diffuse type carcinoma. (c) High Ki-67 index (>20%) in diffuse type carcinoma

|?Figure.3Correlation between CDX2 and Ki-67
References
- osman FT, Carneiro F, Hruban RH, Theise ND.?WHO Classification of Tumors of the Digestive System. Lyon: IARC 2010; 4: 48-58
- arkin DM.?International variation. Oncogene 2014; 23: 6329-34
- erlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM.?Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893-917
- auren P, Sarin YK.?The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31-49
- uh E, Chen L, Taylor J, Traber PG.?A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol Cell Biol 1994; 14: 7340-51
- izoshita T, Inada K, Tsukamoto T, Kodera Y, Yamamura Y, Hirai T.?et al.?Expression of CDX1 and CDX2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa with special emphasis on participation in intestinal metaplasia of the human stomach. Gastric Cancer 2001; 294: 470-9
- lmeida R, Silva E, Santos-Silva F, Silberg DG, Wang J, De Bol?sC.?et al.?Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol 2003; 199: 36-40
- atoh K, Mutoh H, Eda A, Yanaka I, Osawa H, Honda S.?et al.?Aberrant expression of CDX2 in the gastric mucosa with and without intestinal metaplasia: Effect of eradication of Helicobacter pylori. Helicobacter 2002; 7: 192-8
- eno H, Oshima M, Taniguchi MA, Usami K, Ishikawa TO, Chiba T.?et al.?CDX2 expression in the stomach with intestinal metaplasia and intestinal-type cancer: Prognostic implications. Int J Oncol 2002; 21: 769-74
- Lazar D, Taban S, Sporea I, Dema A, Cornianu M, Lazar E.?et al.?Ki-67 expression in gastric cancer. Results from a prospective study with long-term follow-up. Rom J Morphol Embryol 2010; 51: 655-61
- Yu J, He Y, Guo Z.?Age trend of the male to female sex ratio in surgical gastric cancer patients at a single institution. World J Surg Oncol 2014; 12: 269
- Janssen CWJr, Maartmann-Moe H, Lie RT, Matre R.?Age and sex distribution of intestinal type and diffuse gastric carcinoma. APMIS 1991; 99: 78-82
- Saha AK, Maitra S, Hazra SC.?Epidemiology of gastric cancer in the gangetic areas of West Bengal. ISRN Gastroenterol 2013; 2013: 823483
- trong>?Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y.?et al.?Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer 2014; 7: 46-53
- Lee YY, Derakhshan MH.?Environmental and lifestyle risk factors of gastric cancer. Arch Iran Med 2013; 16: 358-65
- Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J.?Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: Increase in the signet ring cell type. Arch Pathol Lab Med 2004; 128: 765-70
- Wu H, Rusiecki JA, Zhu K, Potter J, Devesa SS.?Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev 2009; 1945: 52
- Lundeg?rdh G, Lindgren A, Rohul A, Nyr?n O, Hansson LE, Bergstr?m R.?et al?Intestinal and diffuse types of gastric cancer: Secular trends in Sweden since 1951. Br J Cancer 1991; 64: 1182-6
- Cambruzzi E, Azeredo AM, Kronhart A, Foltz KM, Zettler CG, P?gas KL.?The presence of metastases in regional lymph nodes is associated with tumor size and depth of invasion in sporadic gastric adenocarcinoma. Arq Bras Cir Dig 2014; 27: 18-21
- Liu Y, Chen XH, Meng XH, Liu CF, Zhao LL, Han JW.?et al.?Multivariate prognostic study on node-positive gastric cancer: Is tumor size a prognostic indicator?. Hepatogastroenterology 2012; 59: 623-6
- Folli S, Morgagni P, Roviello F, De ManzoniG, Marrelli D, Saragoni l.?et al.?Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol 2001; 31: 495-9
- Ha KimG, Am SongG, Youn ParkD, Han LeeS, Hyun LeeD, Oh KimT.?et al.?CDX2 expression is increased in gastric cancers with less invasiveness and intestinal mucin phenotype. Scand J Gastroenterol 2006; 41: 880-6
- Roessler K, M?nig SP, Schneider PM, Hanisch FG, Landsberg S, Thiele J.?et al?Co-expression of CDX2 and MUC2 in gastric carcinomas: Correlations with clinico-pathological parameters and prognosis. World J Gastroenterol 2005; 11: 3182-8
- Fan Z, Li J, Dong B, Huang X.?Expression of Cdx2 and hepatocyte antigen in gastric carcinoma: Correlation with histologic type and implications for prognosis. Clin Cancer Res 2005; 11: 6162-70
- Ramires M, David L, Leit?o D, Seixas M, Sansonetty F, Sobrinho-Sim?e M.?Ki67 labelling index in gastric carcinomas. An immunohistochemical study using double staining for the evaluation of the proliferative activity of diffuse-type carcinomas. J Pathol 1997; 182: 62-7


 PDF
PDF  Views
Views  Share
Share

