Catch-22 Situation with Unexpected Reports in Acute Lymphoblastic Leukemia
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(02): 211-213
DOI: DOI: 10.1055/s-0045-1801886
Abstract
Central nervous system (CNS) infections are relatively common among children receiving treatment for acute lymphoblastic leukemia (ALL). However, diagnosing these infections presents challenges. In this report, we present a case of asymptomatic adenoviral meningitis, which presented a diagnostic challenge as it mimicked CNS involvement in a child undergoing treatment for ALL. Our findings underscore the importance of thorough diagnostic evaluation for CNS infections in children undergoing ALL therapy, whether they present with symptoms or exhibit asymptomatic cerebrospinal fluid pleocytosis. Furthermore, distinguishing between infections and CNS leukemia is critical, highlighting the necessity of employing flow cytometry to mitigate the potential misinterpretation of morphological features.
Patient Consent
Patient consent is not required in this study.
Author Contributions
S.P. wrote the manuscript, A.T. is the clinician involved in treatment and contributed to betterment of manuscript, P.G. is the Cytopathologist who reported the CSF samples and provided the required images.
Publication History
Article published online:
04 February 2025
© 2025. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- SEIZURES IN CHILDREN WITH ACUTE LYMPHOBLASTIC LEUKEMIAW. Mathiassen, Neuropediatrics, 2006
- SEIZURES IN CHILDREN WITH ACUTE LYMPHOBLASTIC LEUKEMIAW. Mathiassen, Aktuelle Traumatologie, 2006
- Targeting pediatric acute lymphoblastic leukemia with oncolytic measlesAV Goß, Journal of Pediatric Biochemistry
- Pictorial essay: Acute neurological complications in children with acute lymphoblastic leukemiaSeema A Kembhavi, Indian J Radiol Imaging, 2012
- Radiation-induced hypopituitarism in children with acute lymphoblastic leukemiaMehrdad Mirouliaei, Indian Journal of Medical and Paediatric Oncology, 2013
- PERSONALIZATION OF 6-MERCAPTOPURINE THERAPY FOR CHILDREN WITH ACUTE LYMPHOBLASTIC LEUKEMIA<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- PP-058 Postchemotherapy complications in a child with acute lymphoblastic leukemia<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- 1516 Electrophysiological Study of Peripheral Nerves in Children with Acute Lymphoblastic Leukemia<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Poor prognosis of acute lymphoblastic leukemia in non-European children.<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- P567 Leukaemia cutis: a rare manifestation of acute lymphoblastic leukemia<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
Abstract
Central nervous system (CNS) infections are relatively common among children receiving treatment for acute lymphoblastic leukemia (ALL). However, diagnosing these infections presents challenges. In this report, we present a case of asymptomatic adenoviral meningitis, which presented a diagnostic challenge as it mimicked CNS involvement in a child undergoing treatment for ALL. Our findings underscore the importance of thorough diagnostic evaluation for CNS infections in children undergoing ALL therapy, whether they present with symptoms or exhibit asymptomatic cerebrospinal fluid pleocytosis. Furthermore, distinguishing between infections and CNS leukemia is critical, highlighting the necessity of employing flow cytometry to mitigate the potential misinterpretation of morphological features.
Keywords
acute lymphoblastic leukemia - CSF pleocytosis - CNS infection - CNS leukemia.Introduction
A 3-year-old boy presented with fever, and bone pains for the past 3 months. On examination, he had pallor, petechiae, cervical lymphadenopathy, and hepatosplenomegaly. Clinical investigations revealed the following: hemoglobin, 104 g/L; reticulocyte count, 0.28%; platelet count, 11 × 109/L; and white blood cell count, 22.9 × 109/L with 97%. blasts. He was diagnosed with pre-B acute lymphoblastic leukemia (PB-ALL) on flow cytometric immunophenotyping. There were no adverse cytogenetics, and he was risk stratified as a standard risk PB-ALL.[1] He was started on induction phase chemotherapy. A diagnostic lumbar puncture done on day 8 of chemotherapy showed a cell count of 12 cells/mm3 (neutrophils/lymphocytes: 14/86), and the malignant cytology was negative, consistent with central nervous system (CNS) negative disease. Reassessment bone marrow at the end of induction had 3%. blasts. However, the minimal residual disease (MRD) was positive (0.05%), necessitating change to the high-risk arm of therapy. The cerebrospinal fluid (CSF) sample sent for malignant cytology at the end of induction (3rd CSF, intrathecal being administered on days 8, 15, and 35 of induction as per protocol) was reported positive. The child had received multiple intrathecal therapies as depicted in [Table 1], among which the third CSF (end of induction) and the sixth CSF (during consolidation) were reported to be infiltrated by leukemic blasts as shown in [Fig. 1].
|
Sl. no. |
Cell count/cellularity |
CSF—malignant cytology |
Flow cytometry |
|---|---|---|---|
|
1 |
12 (N/L, %: 14/86) |
Negative |
– |
|
2 |
– |
Lymphocytic pleocytosis |
– |
|
3 |
– |
Positive (end of induction) |
– |
|
4 |
Moderately cellular |
Pleocytosis |
– |
|
5 |
30 (N/M, %: 20/80) |
Negative |
– |
|
6 |
– |
Positive (during consolidation) |
– |
|
7 |
Highly cellular |
Inflammation, no malignant cells |
15% monocytes, 77% lymphocytes (45 |
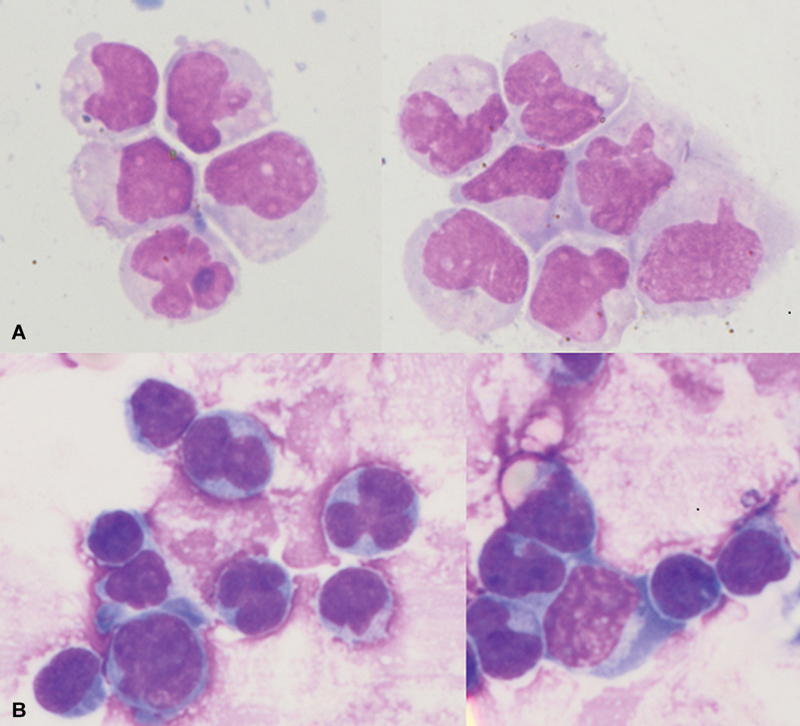
Fig 1: (A)Cerebrospinal fluid (CSF) cytology shows scattered atypical blasts having high nucleus-to-cytoplasm'' ratio (N:C ratio) with opened-up nuclear chromatin, occasional prominent nucleoli, and scant amount of cytoplasm. (B) CSF cytology shows infiltration by blasts with high N:C ratio, inconspicuous nuclei, and scant amount of pale basophilic granular cytoplasm.
The CSF samples showed pleocytosis (predominantly had monocytosis and activated lymphocytes—morphologically mimicking ALL blasts). The possibility of infective/chemical meningitis was considered. However, the patient was asymptomatic during the entire period. A detailed molecular investigation of the CSF, which included cell count, Gram stain/culture, flow cytometry, polymerase chain reaction (PCR) for detection of herpes simplex virus (HSV) DNA, cytomegalovirus (CMV) PCR, adenovirus PCR, Japanese encephalitis (JE) PCR, and enterovirus PCR, was performed. The CSF had a cell count of 491 cells/mm3 (predominantly had monocytosis and activated lymphocytes), the sugar/protein levels were normal (normal glucose level is 50–80 mg/dL, normal protein level is 20–45 mg/dL in the CSF), Gram stain negative, and the culture was sterile. Flow cytometry revealed no blasts—CD45+ lymphocytes were negative for CD10, CD19, and CD34. The results for HSV PCR, CMV PCR, JE PCR, and enterovirus PCR were negative. However, the result for adenovirus PCR was found to be positive. Pleocytosis was attributed to adenovirus meningitis, and the child was continued on therapy as a case of CNS-negative ALL. The child completed consolidation and interim maintenance phases of chemotherapy uneventfully. Unfortunately, he developed fever with loose stools following delayed-intensification therapy and succumbed to possible gram-negative sepsis.
Discussion
CNS involvement at diagnosis in childhood ALL ranges from 3 to 5% in B-ALL and 10 to 15% in T-ALL.[2] [3] [4] Being a sanctuary site, CNS-directed prophylaxis is given to all children with ALL. Patients who have CNS disease at diagnosis receive intensive CNS-directed therapy, which may include varying combinations of intrathecal chemotherapy, systemic chemotherapy with high-dose methotrexate (HD-MTX), or Capizzi escalating MTX with Peg-asparaginase, often coupled with cranial irradiation to improve survival.[5] [6]
CNS-3 leukemia is often asymptomatic at diagnosis, although some patients present with symptoms such as headache, nausea, vomiting, lethargy, irritability, nuchal rigidity, papilledema, and cranial nerve deficits.[7] CNS infections in immunocompromised children can range from asymptomatic presentations (our index case) to severe symptomatic cases.[2]
Distinguishing CNS infection from CNS leukemia is clinically challenging. CSF cell counts are typically elevated in both scenarios, with neutrophilic predominance observed in bacterial infections and lymphocytic predominance in viral infections and CNS leukemia. Cytomorphology alone may be misleading, as activated lymphocytes and monocytes in viral infections can resemble ALL blasts, potentially resulting in false CNS-positive labeling. Hence, comprehensive infectious workup, including cultures and PCR, are essential for identifying infectious etiologies; however, serology may yield false negatives in immunocompromised patients. CSF flow cytometry with timely processing is crucial for confirming the presence of leukemic blasts in CNS-3 leukemia.[8]
In the index case, as there was clinicopathological discorrelation, efforts were made to confirm the CNS status. Eventually, it was confirmed as negative on repeat morphology and flow cytometry. An extensive infectious workup was done to determine the etiology of the pleocytosis wherein adenovirus PCR was positive. Human adenovirus (HAdv) infections are mostly asymptomatic. Meningoencephalitis is a rare complication of HAdv infection, and it is usually seen in immunocompromised patients. Neurological manifestations range from mild aseptic meningitis as seen in our patient to potentially fatal acute necrotizing encephalopathy.[9]
Conclusion
This case highlights the difficulties faced by the cytopathologist and the dilemma of the clinician in the interpretation and management of a discordant CSF cytology report with repeated pleocytosis. Activated lymphocytes mimic ALL blasts leading to false-positive labeling of the acquired CSF sample. In case of doubt/discrepancy, CSF flow cytometry[8] should always be performed along with cytomorphological diagnosis to confirm the findings, as shown for our index patient since infectious cases necessarily require intensive investigation.
Conflict of Interest
None declared.
Patient Consent
Patient consent is not required in this study.
Author Contributions
S.P. wrote the manuscript, A.T. is the clinician involved in treatment and contributed to betterment of manuscript, P.G. is the Cytopathologist who reported the CSF samples and provided the required images.
References
- Das N, Banavali S, Bakhshi S. et al. Protocol for ICiCLe-ALL-14 (InPOG-ALL-15-01): a prospective, risk stratified, randomised, multicentre, open label, controlled therapeutic trial for newly diagnosed childhood acute lymphoblastic leukaemia in India. Trials 2022; 23 (01) 102
- Blaney SM, Adamson PC, Helman LJ. Pizzo and Poplack's Pediatric Oncology. Vol. 1. 8th ed.. Philadelphia, PA: Wolters Kluwer; 2021
- Winick N, Devidas M, Chen S. et al. Impact of initial CSF findings on outcome among patients with national cancer institute standard- and high-risk b-cell acute lymphoblastic leukemia: a report from the Children's Oncology Group. J Clin Oncol 2017; 35 (22) 2527-2534
- Schultz KR, Pullen DJ, Sather HN. et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG). Blood 2007; 109 (03) 926-935
- Larsen EC, Salzer WL, Devidas M. et al. Comparison of high-dose methotrexate (HD-MTX) with Capizzi methotrexate plus asparaginase (C-MTX/ASNase) in children and young adults with high-risk acute lymphoblastic leukemia (HR-ALL): a report from the Children's Oncology Group Study AALL0232. JCO 2011; 29 (18, suppl): 3
- Jeha S, Pei D, Choi J. et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol 2019; 37 (35) 3377-3391
- Marwaha RK, Kulkarni KP, Bansal D, Trehan A. Central nervous system involvement at presentation in childhood acute lymphoblastic leukemia: management experience and lessons. Leuk Lymphoma 2010; 51 (02) 261-268
- Thastrup M, Marquart HV, Levinsen M. et al; Nordic Society of Pediatric Hematology and Oncology (NOPHO). Flow cytometric detection of leukemic blasts in cerebrospinal fluid predicts risk of relapse in childhood acute lymphoblastic leukemia: a Nordic Society of Pediatric Hematology and Oncology study. Leukemia 2020; 34 (02) 336-346
-
Shieh WJ. Human adenovirus infections in pediatric population - An update on clinico-pathologic correlation. Biomed J 2022; 45 (01) 38-49
Address for correspondence
Amita Trehan, MDDivision of Pediatric Hematology-Oncology, Department of Pediatrics, Advanced Pediatric Centre, Postgraduate Institute of Medical Education and ResearchChandigarh 160012IndiaEmail: trehanamita@hotmail.comPublication History
Article published online:
04 February 2025© 2025. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, IndiaWe recommend- SEIZURES IN CHILDREN WITH ACUTE LYMPHOBLASTIC LEUKEMIAW. Mathiassen, Aktuelle Traumatologie, 2006
- SEIZURES IN CHILDREN WITH ACUTE LYMPHOBLASTIC LEUKEMIAW. Mathiassen, Neuropediatrics, 2006
- Targeting pediatric acute lymphoblastic leukemia with oncolytic measlesAV Goß, Journal of Pediatric Biochemistry
- Pictorial essay: Acute neurological complications in children with acute lymphoblastic leukemiaSeema A Kembhavi, Indian J Radiol Imaging, 2012
- Acute Lymphoblastic Leukemia at Birth with KMT2A Gene RearrangementSanjeev Kumar Jha, South Asian Journal of Cancer
- Data-driven estimation of economic indicators with search big data in discontinuous situation<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Unexplained Sets of Seismographic Station Reports and a Set Consistent with a Quark Nugget Passage<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Soleus arthrogenic muscle inhibition following acute lateral ankle sprain correlates with symptoms and ankle disability but not with postural control<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Revisiting the stretch-induced force deficit: A systematic review with multilevel meta-analysis of acute effects<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Comment on “Unexplained Sets of Seismographic Station Reports and a Set Consistent with a Quark Nugget Passage” by David P. Anderson, Eugene T. Herrin, Vigdor L...<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- SEIZURES IN CHILDREN WITH ACUTE LYMPHOBLASTIC LEUKEMIA

Fig 1: (A)Cerebrospinal fluid (CSF) cytology shows scattered atypical blasts having high nucleus-to-cytoplasm'' ratio (N:C ratio) with opened-up nuclear chromatin, occasional prominent nucleoli, and scant amount of cytoplasm. (B) CSF cytology shows infiltration by blasts with high N:C ratio, inconspicuous nuclei, and scant amount of pale basophilic granular cytoplasm.
References
- Das N, Banavali S, Bakhshi S. et al. Protocol for ICiCLe-ALL-14 (InPOG-ALL-15-01): a prospective, risk stratified, randomised, multicentre, open label, controlled therapeutic trial for newly diagnosed childhood acute lymphoblastic leukaemia in India. Trials 2022; 23 (01) 102
- Blaney SM, Adamson PC, Helman LJ. Pizzo and Poplack's Pediatric Oncology. Vol. 1. 8th ed.. Philadelphia, PA: Wolters Kluwer; 2021
- Winick N, Devidas M, Chen S. et al. Impact of initial CSF findings on outcome among patients with national cancer institute standard- and high-risk b-cell acute lymphoblastic leukemia: a report from the Children's Oncology Group. J Clin Oncol 2017; 35 (22) 2527-2534
- Schultz KR, Pullen DJ, Sather HN. et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG). Blood 2007; 109 (03) 926-935
- Larsen EC, Salzer WL, Devidas M. et al. Comparison of high-dose methotrexate (HD-MTX) with Capizzi methotrexate plus asparaginase (C-MTX/ASNase) in children and young adults with high-risk acute lymphoblastic leukemia (HR-ALL): a report from the Children's Oncology Group Study AALL0232. JCO 2011; 29 (18, suppl): 3
- Jeha S, Pei D, Choi J. et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol 2019; 37 (35) 3377-3391
- Marwaha RK, Kulkarni KP, Bansal D, Trehan A. Central nervous system involvement at presentation in childhood acute lymphoblastic leukemia: management experience and lessons. Leuk Lymphoma 2010; 51 (02) 261-268
- Thastrup M, Marquart HV, Levinsen M. et al; Nordic Society of Pediatric Hematology and Oncology (NOPHO). Flow cytometric detection of leukemic blasts in cerebrospinal fluid predicts risk of relapse in childhood acute lymphoblastic leukemia: a Nordic Society of Pediatric Hematology and Oncology study. Leukemia 2020; 34 (02) 336-346
- Shieh WJ. Human adenovirus infections in pediatric population - An update on clinico-pathologic correlation. Biomed J 2022; 45 (01) 38-49


 PDF
PDF  Views
Views  Share
Share

