Burden of cervical cancer and role of screening in India
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(04): 278-285
DOI: DOI: 10.4103/0971-5851.195751
Abstract
Background: Cervical cancer is a major cause of cancer mortality in women and more than a quarter of its global burden is contributed by developing countries. In India, in spite of alarmingly high figures, there is no nationwide government-sponsored screening program. This study was conducted to assess the burden of cervical cancer in India and review the performance characteristics of available cervical cancer screening tools, so as to provide evidence-based recommendations for application of most practically suited screening test to be used in resource-poor field settings. Materials and Methods: MEDLINE and Web of Science electronic database were searched from January 1990 to December 2015, using the keywords such as “cervical cancer”, “screening”, “early detection”, “cervical cytology” and “visual inspection”, and their corresponding MeSH terms in combination with Boolean operators “OR, AND.” Two authors independently selected studies that are published in English and conducted in India. A total of 11 studies were found to be relevant and eligible to be included in the present study. Results: In India, cervical cancer contributes to approximately 6–29% of all cancers in women. The age-adjusted incidence rate of cervical cancer varies widely among registries; highest is 23.07/100,000 in Mizoram state and the lowest is 4.91/100,000 in Dibrugarh district. The pooled estimates of sensitivity and specificity of visual inspection with acetic acid (VIA), magnified VIA, visual inspection with Lugol's iodine (VILI), cytology (Pap smear), and human papillomavirus DNA were found to be 67.65% and 84.32%, 65.36% and 85.76%, 78.27% and 87.10%, 62.11% and 93.51%, and 77.81% and 91.54%, respectively. Conclusions: In developing countries because of lack of necessary infrastructure and quality control, high-quality cytology screening may not be feasible for wide-scale implementation. Hence, cervical cancer screening program based on visual screening test such as VIA/VILI should be adopted as an integral part of primary health-care setup in resource-poor countries like India.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Cervical cancer is a major cause of cancer mortality in women and more than a quarter of its global burden is contributed by developing countries. In India, in spite of alarmingly high figures, there is no nationwide government-sponsored screening program. This study was conducted to assess the burden of cervical cancer in India and review the performance characteristics of available cervical cancer screening tools, so as to provide evidence-based recommendations for application of most practically suited screening test to be used in resource-poor field settings.
Materials and Methods:
MEDLINE and Web of Science electronic database were searched from January 1990 to December 2015, using the keywords such as “cervical cancer”, “screening”, “early detection”, “cervical cytology” and “visual inspection”, and their corresponding MeSH terms in combination with Boolean operators “OR, AND.” Two authors independently selected studies that are published in English and conducted in India. A total of 11 studies were found to be relevant and eligible to be included in the present study.
Results:
In India, cervical cancer contributes to approximately 6–29% of all cancers in women. The age-adjusted incidence rate of cervical cancer varies widely among registries; highest is 23.07/100,000 in Mizoram state and the lowest is 4.91/100,000 in Dibrugarh district. The pooled estimates of sensitivity and specificity of visual inspection with acetic acid (VIA), magnified VIA, visual inspection with Lugol's iodine (VILI), cytology (Pap smear), and human papillomavirus DNA were found to be 67.65% and 84.32%, 65.36% and 85.76%, 78.27% and 87.10%, 62.11% and 93.51%, and 77.81% and 91.54%, respectively.
Conclusions:
In developing countries because of lack of necessary infrastructure and quality control, high-quality cytology screening may not be feasible for wide-scale implementation. Hence, cervical cancer screening program based on visual screening test such as VIA/VILI should be adopted as an integral part of primary health-care setup in resource-poor countries like India.
INTRODUCTION
Cancer is one of the leading causes of adult deaths worldwide. Every year about 14 million new cancer cases are detected and 8 million people die of cancer.[1] However, there is a marked difference in the distribution of cancer sites across different regions of the world. In contrast to developed countries, cervical cancer is a public health problem in developing countries like India, so much so that India alone accounts for one-quarter of the worldwide burden of cervical cancers.[1,2] It is the one of the leading cause of cancer mortality, accounting for 17% of all cancer deaths among women aged between 30 and 69 years. It is estimated that cervical cancer will occur in approximately 1 in 53 Indian women during their lifetime compared with 1 in 100 women in more developed regions of the world.[2]
Screening for cancer is known to reduce mortality by early detection and treatment. However, there are two prerequisites for screening to reduce the rate of death from cancer. First, screening must advance the time of diagnosis of cancers that are destined to cause death. Second, early treatment of these cancers must confer some advantage over treatment at clinical presentation.[3,4] Unlike other cancer sites, cervix can be subjected to screening for early diagnosis and treatment. However, despite availability of various cervical cancer screening methods, as well as large burden of disease in India, there is no countrywide government-sponsored public health policy on prevention of cervical cancer by either screening or vaccination or both. Therefore, this study was carried out to understand and present burden of cervical cancer in India, as well as to appraise the various cervical cancer screening methods and studies conducted for evaluating screening test for the detection of cervical carcinoma. However, since India is culturally, economically, and sociodemographically dissimilar from other Western countries, we limited the scope of our study to screening trials conducted in Indian population, so as to provide locally relevant evidence-based recommendations for cervical cancer screening in Indian population.
MATERIALS AND METHODS
Data sources and searches
This paper is based on information gathered from a review of peer-reviewed publications on cervical cancer screening and prevention in India. MEDLINE (http://www.pubmed.com) and Web of Science electronic database were searched from January 1990 to December 2015 using the keywords such as “cervical cancer”, “screening”, “early detection”, “human papillomavirus (HPV)”, “cervical cytology” and “visual inspection”, and their corresponding MeSH terms were also used in combination with Boolean operators “OR, AND.” We also examined bibliographies of included articles to identify additional references. The search strategy was limited to English language. Only journal article type was included. Figure 1 presents the search strategy and screening process.
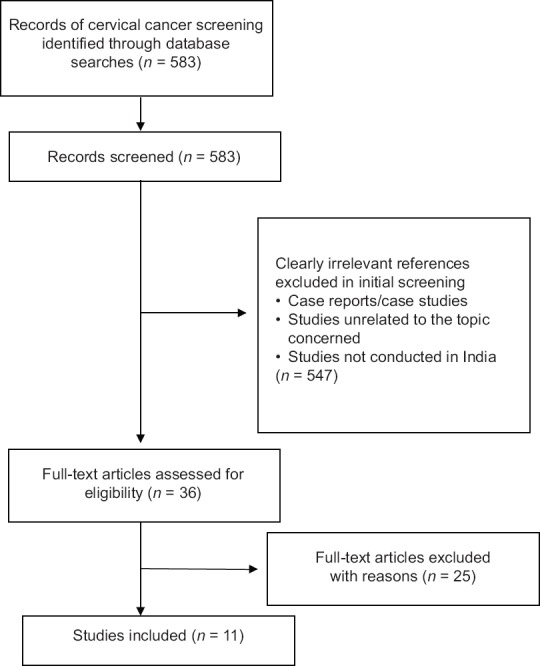
| Fig. 1 Summary of evidence search and selection
Study selection
Inclusion criteria
- Study designs eligible for inclusion in our study were randomized controlled trials, nonrandomized controlled trials, cohort studies, and cross-sectional studies conducted to evaluate the performance of the screening tests for detection of cervical cancer
- Studies conducted in India only were included in the study.
Exclusion criteria
- Studies not providing data on sensitivity and specificity of screening test were evaluated and excluded from the study.
Data extraction and analysis
The title and abstract of each citation were screened first, and full report was screened second if necessary to select the relevant articles according to selection criteria. Full texts of these selected studies were retrieved, reviewed, and extracted for relevant data by authors independently. A total of 11 studies were included in the review, and their findings have been presented. The descriptive statistical data for cervical cancer (International Classification of Diseases, Tenth Edition, C53) were mainly obtained from publications of the Indian Cancer Registry, Union for International Cancer Control, International Agency for Research on Cancer, and WHO (GLOBOCAN-2012).
RESULTS
Descriptive epidemiology
The National Cancer Registry Programme (NCRP) has been in existence since 1982, and permanent institute (National Centre for Disease Informatics and Research) of the Indian Council of Medical Research is the crucial repository of data from the collaborating cancer registries located in medical colleges/institutions and hospitals throughout India. As of March 2016, there are 29 hospital-based cancer registries (including all Regional Cancer Centres) and 29 population-based cancer registries (PBCRs) under NCRP.[5,6] The data obtained from these Indian Cancer Registries indicate that cervical cancer contributes to approximately 6–29% of all cancer in females. The age-adjusted incidence rate of cancer cervix was found to vary widely among registries, highest being 23.07/100,000 in Mizoram State, followed by 22.54/100,000 in Pasighat and the lowest being 4.91/100,000 in Dibrugarh district. The older PBCRs such as Bengaluru, Bhopal, Chennai, Delhi, and Barshi Rural reported an age-adjusted incidence rate between 13 and 16/100,000 [Table 1].[5] All the major registries from 1998 to 2014 reported a statistically significant decreasing trend (negative annual percentage change) of cervical cancer [Table 2].[5] More than 85% of patients in were from age group 40 years and above. The maximum numbers of cases were reported in 50–59 years of age group amounting to 27.37% of all cervical carcinoma cases [Table 3].[5]
Table 1
Age-adjusted (world) incidence rates of cervix uteri cancer* and its relative proportion in Indian population-based cancer registries
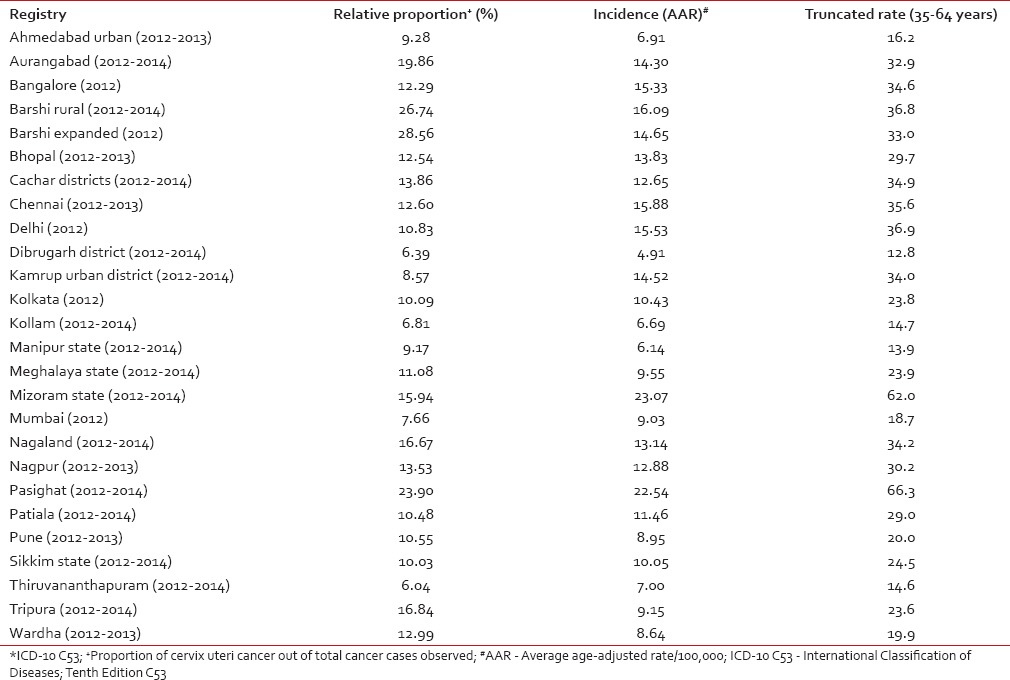
Table 2
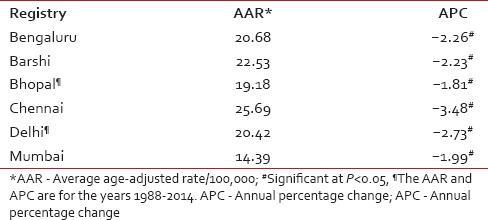
Table 3
Age-wise distribution of cervix uteri cancer

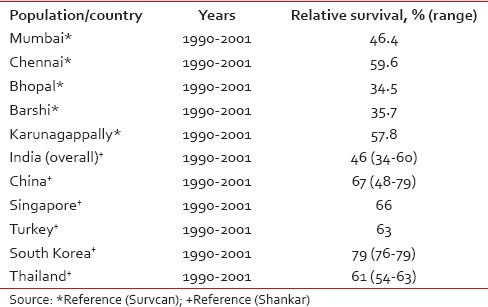
The 5-year relative survival rate for cancer cervix in India has been reported to be approximately 46% (34–60),[7] which much lesser than survival rates reported from other Asian countries such as China, South Korea, Singapore, and Thailand [Table 4]. In addition, cervical cancer survival rates in India also shows a wide variation ranging from 59.6% in Chennai to 34.5% in Bhopal. The 5-year age-standardized relative survival of cervical cancer for different Indian registries and its comparison with other Asian registries are shown in Table 4.[7,8]
Table 4
Five-year age-standardized (0–74 years) relative survival for cervix uteri cancer in selected Indian populations and selected Asian countries
Cervical cancer screening
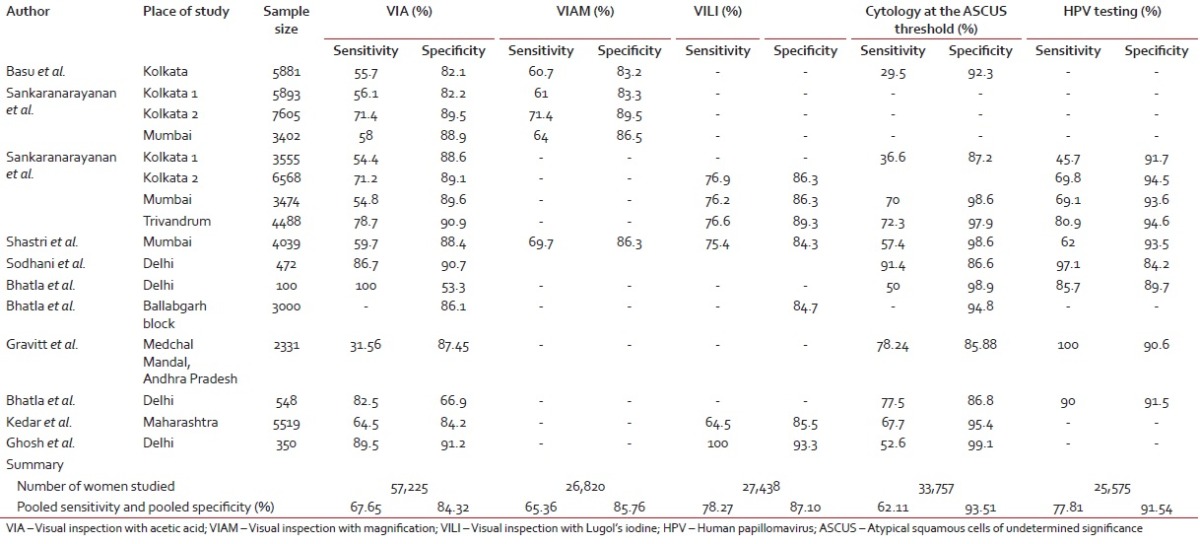
Cervix is amenable to screening by a number of methods which include visual inspection with acetic acid (VIA), magnified VIA (VIAM) visual inspection with Lugol's iodine (VILI), the Papanicolaou test, and HPV DNA testing. A brief overview including strengths and weaknesses of each screening modality is presented in Table 5.[9,10,11,12,13,14,15] Salient findings of Indian studies[16,17,18,19,20,21,22,23,24,25,26] on screening test performance are summarized in Table 6. The pooled sensitivity and specificity for VIA was found to be 67.65% and 84.32%, for VIAM, the pooled sensitivity and specificity were 65.36% and 85.76% and for VILI, the pooled sensitivity and specificity were 78.27% and 87.10%, respectively. The pooled sensitivity and specificity for cytology positivity at low-grade squamous intraepithelial neoplasia threshold were 62.11% and 93.51% and for HPV, the pooled sensitivity and specificity were 77.81% and 91.54%, respectively.
Table 5
Overview of primary screening tools for cervical cancer
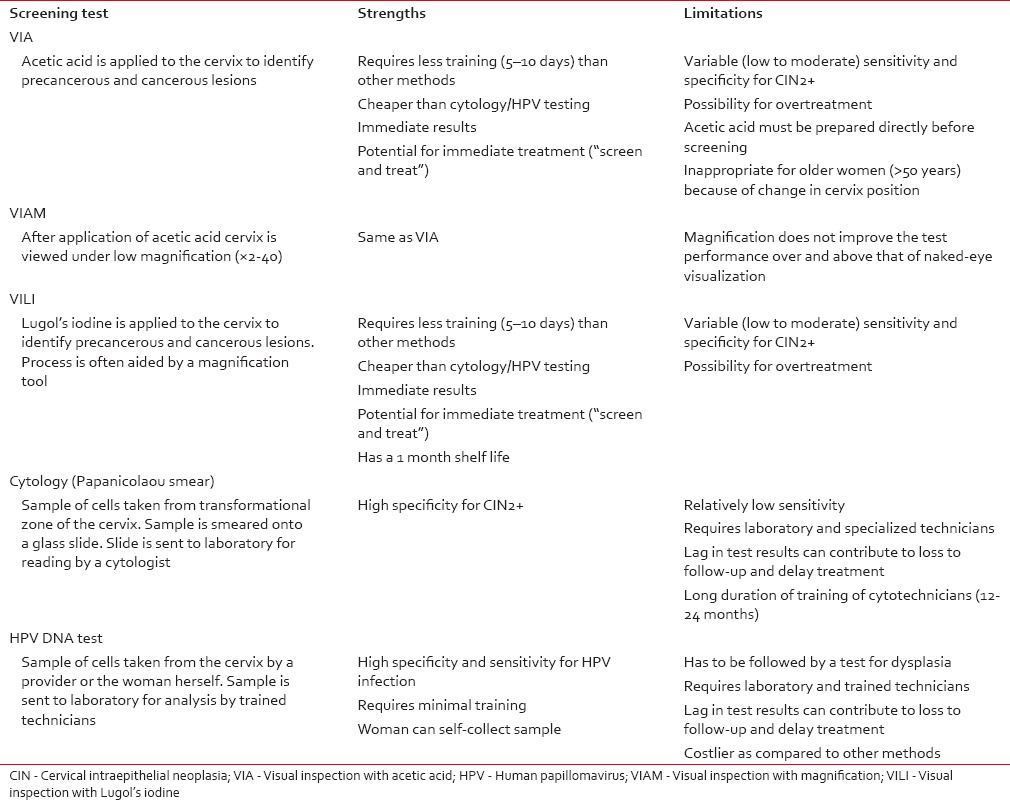
Table 6
DISCUSSION
In today's era, in spite of the availability of HPV vaccines and affordable and effective methods for early detection and treatment of cervical cancer precursor lesions, cervical cancer still continues to be a public health problem in India. The age-adjusted incidence rates of cancer cervix reported by majority of Indian cancer registries are much higher than the world age-adjusted incidence rate of 7.9/100,000 population but is lower or similar to cervical cancer incidence rates of 19.2/100,000 population seen in the South-East Asian region.[1] The high burden of cervical cancer in India and Southeast Asian countries is due to poor to moderate living standards, a high prevalence of HPV (more than 10% in women aged more than 30 years) and due to lack of screening.[27] There is a wide variation (range: 4.91–23.07/100,000) in the incidence rates reported by individual PBCRs [Table 1], more so these extreme rates have been reported by newer registries. This is because some PBCRs are reporting the data for the first time and hence, it needs to be viewed with some caution as in the initial years of registry operation there could be some degree of over/under reporting.[5]
Most of the major registries have shown a decreasing trend of cervical cancer, however, the decrease was very small. The mean annual percentage decrease in the average age-adjusted rate ranged from 1.81% to 3.48% among various registries. It should be noted that in India most of the cervical cancer cases are detected with regional spread of the disease, and a very small proportion are diagnosed at a localized stage. In India, an organized mass-screening program for early detection of cervical cancer is not in practice; hence, this decrease cannot be attributed to screening.[28] Moreover, it is also highly probable that cervical cancer incidence rates are an underestimate for India possibly due to underdiagnosis of cervical cancer cases in rural areas and among most impoverished women, as well as due to noninclusion of subclinical cervical cancers in routine hysterectomy specimens not subjected to histopathology, which is a common practice in many regions of India.[29] On the other hand, it has also been suggested that probably an improvement in the living standard of women may have resulted in a reduction in the incidence of cervical cancer, but the same has not been substantiated.[30] The 5-year age-standardized (0–74 years) relative survival for cervical cancer in India is much lower as compared to certain South East Asian countries [Table 4]. This can be attributed to the absence of a nationwide screening program as survival is determined by age and the extent of disease, with younger women having longer survival.[31] A wide variation of survival rates is also observed between rural and urban India. This could be because Indian registries suffer with the problem of under-reporting of death, which is more pronounced in the rural area. In addition, the lack of health infrastructure in rural India results in lower survival rates as compared to urban areas.
In India, despite such alarming statistics there has been no synchronized initiative from public health authorities for prevention and control cervical cancer. In developed countries, conventional cytology screening programs have shown a marked decline in the incidence of cervical cancer.[27,32] However, its successful implementation requires a variety of requirements to be fulfilled, which are not feasible in many low-resource settings where a high risk of cervical cancer is experienced. For instance, cytology screening requires a laboratory infrastructure, microscopes, several resource personnel (smear collectors, cytotechnicians, and pathologists), consumables (slides, fixative, Pap stain containing five dyes, and three solutions), and several steps with inbuilt quality assurance procedures. Moreover, cytology must be repeated at frequent 3–5-year intervals to ensure satisfactory sensitivity and optimum detection of cervical cancer precursor lesions and repeat visits are necessary after a positive cytology for diagnosis and treatment, which may lead to drop outs. Several years of cytology screening in low- and middle-income countries have not led to significant reductions in cervical cancer in these countries, possibly due to the difficulties in offering high-quality cytology, programmatic deficiencies in follow-up and treatment of screen-positive women, and also due to the considerable financial, technical, and logistic inputs necessary for effective cytology programs.[25,33] Hence, these challenges have prompted the search for programs based on alternative cervical screening tests, which are implementable in resource-poor settings. VIA is one of the alternative methods for cervical cancer screening that has been widely investigated. In our review of Indian studies, the sensitivity of VIA has been found to be better than cytology, but its specificity is lower [Table 4].[16,17,18,19,20,21,22,23,24,25,26] However, the biggest advantage of visual tests is that it can be implemented through primary health-care workers, it does not require a laboratory infrastructure, and the results are obtained immediately following testing, allowing diagnosis and treatment to be instituted during the same visit. It has been well established that cervical neoplasia is caused by persistent infection with certain oncogenic types of HPV. Indian studies of testing for HPV DNA indicate higher sensitivity and comparable specificity as compared to visual inspection and cytology. However, the requirement of sophisticated laboratory infrastructure and high cost make it impracticable to be implementable in resource-poor, low-income countries.
The main objective of any screening program is a reduction in mortality. The efficacy of screening by VIA in reducing the incidence of and mortality from cervical cancer has been investigated through cluster randomized controlled trial in Dindigul (India), a single round of VIA-based screening led to a 25% reduction in cervical cancer incidence and a 35% reduction in mortality over 7 years of follow-up.[34] Similarly, a randomized controlled trial carried out in Maharashtra showed a 31% reduction in cervical cancer mortality in the screening group (mortality rate ratio relative risk = 0.69; 95% confidence interval: 0.54-0.88; P = 0.003) as compared to the control group.[35]
CONCLUSION
The studies conducted in India provide sufficient evidence that cervical cancer screening through simple test like VIA/VILI is affordable, feasible, and an accurate tool for implementation in all health-care settings. In addition, VIA/VILI also provides an opportunity to adopt “see and treat” approach, which is very useful in resource-poor countries where follow-up is poor. These tests can also be easily taught to grass root health workers, who can help in conducting the screening program in remote areas. However, for any cervical screening program to be successful in addition to the use of a reliable and accurate screening test, high rates of coverage and the ability to effectively provide treatment to test positive women are very important. Hence, the development of health services and generation of community involvement are keys to the initiative in reducing the burden of cervical cancer. Our study highlights the success of visual screening tool in early detection and mortality reduction of cervical cancer in a resource-poor setting and thus, provides a unique opportunity for developing countries to integrate screening of cervical neoplasia in primary health-care settings.
Financial support and sponsorship
Nil.
Conflicts of interest

| Fig. 1 Summary of evidence search and selection


 PDF
PDF  Views
Views  Share
Share

