Breast relapse after metastatic alveolar rhabdomyosarcoma: Is it an incurable entity?
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(02): 119-121
DOI: DOI: 10.4103/0971-5851.180139
Abstract
Metastatic breast disease is a very rare condition in children. Rhabdomyosarcoma (RMS) is the most common solid primary tumor in children, but only a few cases of breast metastases have been described. We present the case of a young female with a primary pelvic metastatic alveolar RMS, which metastasized to the breast twice and achieved prolonged complete remission with a multimodal approach.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Metastatic breast disease is a very rare condition in children. Rhabdomyosarcoma (RMS) is the most common solid primary tumor in children, but only a few cases of breast metastases have been described. We present the case of a young female with a primary pelvic metastatic alveolar RMS, which metastasized to the breast twice and achieved prolonged complete remission with a multimodal approach.
INTRODUCTION
Metastatic breast disease represents 1.2-6% of all breast tumors in pediatrics. RMS is the most common solid primary tumor in children, but only a few cases of breast metastases have been described.[1,2]
We present the case of a young female with primary pelvic alveolar rhabdomyosarcoma (RMS) which metastasized to the breast.
CASE REPORT
In April 2000, a 14-year-old female presented with a lump in her right breast affecting the mammary surface and appearing as a violaceus mass.
Eighteen months prior, she was diagnosed with Stage IV right pelvic alveolar RMS [Figure 1] with metastatic lymphadenopathies in the inguinal, iliac, retroperitoneal, mediastinal, and left supraclavicular regions. She was then treated according to the current protocol (MMT-98). This protocol included: IVA (ifosfamide, vincristine, actinomycin D) × 1 cycle, VINCAEPI × 1 cycle (vincristine, carboplatin, epiadriamycin), followed by a high-dose sequential monotherapy schedule incorporating cyclophosphamide, etoposide, and carboplatin with autologous stem cell rescue.
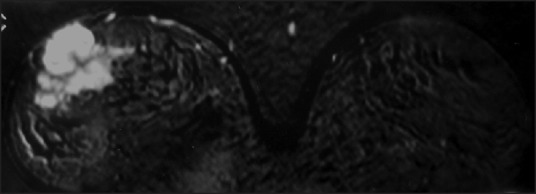
| Fig. 1 Magnetic resonance and T1-weighted images showing heterogeneous lesion with early enhancement and fast wash out in the right breast
She achieved complete response (CR) in primary and metastatic regions and then received maintenance chemotherapy with VAC × 9 cycles (vincristine, actinomicina D, and cyclophosphamide). Concomitant irradiation was administered to the initial affected fields (41, 4 Gy, 180 cGy/day) and boost to the primary tumor (14 Gy, 200 cGy/day) No surgery was performed, due to the inaccessible primary localization.
At the time of the breast lump, our patient was off treatment for 5 months. Considering her previous history, a metastatic relapse was suspected. Magnetic resonance imaging (MRI) showed bilateral heterogeneous lesions as well as lymph nodes in the right axillary region [Figures [Figures11 and and2].2]. A gross needle biopsy detected RMS cells. Further evaluation for recurrent disease at other sites was negative.
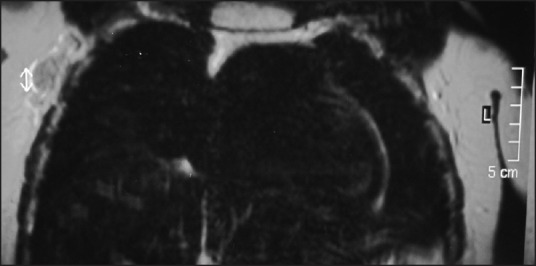
| Fig. 2 Magnetic resonance and T1-weighted images showing enhance lymph nodes in the right axillary region
She then commenced second line chemotherapy with alternating cycles of topotecan (0.75 mg/m2 for 5 days) and cyclophosphamide (250 mg/m2 for 5 days) in 21-day intervals. After 2 cycles, partial response was noted in both breast masses, with CR in axillary region. At this point, the multidisciplinary breast cancer board strongly suggested local radiotherapy based on the argument that all the fields previously irradiated were free of disease at relapse. However, this proposal was rejected by the radiotherapy service who argued about the overlap of the previously irradiated fields and the potential risk of breast irradiation in such a young female.
Instead of radiotherapy, the board decided a radical subcutaneous bilateral mastectomy with dissection of axillary lymph nodes followed by an immediate breast reconstruction as a local treatment. The surgical procedure was successfully performed 6 months after relapse.
After surgery, she was given four cycles of the previous chemotherapeutic regimen as a maintenance therapy, which was discontinued after the fourth cycle because of acute hemorrhagic cystitis. Subsequent evaluation showed CR.
Three months after finishing treatment, she noticed once again, a new subcutaneous mass in her right breast. MRI showed multiple nodular lesions in this location. She was diagnosed as having a second isolated metastatic relapse in the right breast. At this point, the prognosis was considered very poor and in agreement with the family, we decided to start palliative treatment. Six cycles 3 weeks apart combining topotecan and etoposide orally were administered. Consolidation with local radiotherapy was again considered and agreed, at a total of 50 Gy to the right mammary volume.
The assessment with MRI and positron emission tomography at the end of treatment showed CR. The patient remains disease-free for 12 years after finishing treatment with a good quality of life.
DISCUSSION
Children presenting breast metastases from RMS are usually females in postmenarcheal age due to the greater vascularity of the breast, being the limbs and the buttock the most common primary sites with alveolar phenotype.[2]
Although the occurrence of breast metastases is a rare phenomenon, early diagnosis and treatment may have a major impact on prognosis/outcome. Therefore, it seems appropriate to place special attention on the subgroup of risk patients for breast metastases, not only at the time of diagnosis but also during the follow-up. This must include breast examinations and investigation of any suspicious lesions detected. Physical examination and ultrasound may be useful as a first approach, but MRI is considered to be the gold standard.[3]
The overall survival is generally < 30% of patients with Stage IV RMS; but patients having unfavorable prognostic factors at initial diagnosis like alveolar or undifferentiated histology, multiple metastases, age more than 10 years, unfavorable primary location (defined as pelvis, extremities, spine and bone marrow involvement), have an even worse outcome.[4,5,6]
Among the predictive survival factors after recurrence, the alveolar pattern associated with IRS Groups 2, 3, and 4 showed the worst prognosis with a 5 years survival rate approaching 3%.[7]
Although RMS is generally a highly chemosensitive tumor, local treatment plays an essential role in achieving the cure, because local progression or relapse is the major cause of treatment failure. Mutilating surgery is not usually recommended as an initial approach, but can be useful as salvage treatment when other procedures have failed.
Radiotherapy has been long considered as having an important role in local control of childhood RMS. Early evidence suggests that the newer methods of radiation therapy, including intensity-modulated radiotherapy, proton beam radiotherapy, and brachytherapy, may reduce long-term sequelae without affecting efficacy, in comparison with three-dimensional conformal radiotherapy.[8]
Therapy of breast metastases of RMS in children is a challenge, mainly because it is unclear if the criteria commonly used for breast tumors in adults, may be adopted in the pediatric population. Particularly, there is no consensus regarding conservative surgery, and there is also a concern about irradiation of mammary area in young women due to the risk of a secondary tumor.[9]
We conclude that although breast metastases in RMS are very rare, we must consider them in female patients with risk factors.[10] Although treatment is a challenge for the clinician, multimodal strategies might achieve prolonged or complete remission. Radiotherapy still plays an important role in RMS. Newer methods of delivering radiotherapy which potentially minimize side effects must be taken into account.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.

| Fig. 1 Magnetic resonance and T1-weighted images showing heterogeneous lesion with early enhancement and fast wash out in the right breast

| Fig. 2 Magnetic resonance and T1-weighted images showing enhance lymph nodes in the right axillary region


 PDF
PDF  Views
Views  Share
Share

