B-Lymphoblastic Leukemia Presenting with an Isoderivative Philadelphia Chromosome—A Rare Case Report and Review of Literature
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(01): 109-113
DOI: 10.1055/s-0043-1770094
Abstract
The Philadelphia chromosome is seen in 5%, of pediatric and 25 to 50%, of adult cases of acute lymphoblastic leukemia (ALL). It is linked to aggressive illness with a dismal prognosis. Additional chromosomal abnormalities are not prevalent with translocation 9;22; nevertheless, isochromosome derivative [ider(22)] with this translocation is rarely recorded in the literature. This is the third instance of ider(22) in pediatric B-cell acute lymphoblastic leukemia (B-ALL). Bone marrow chromosome analysis by G-banding showed 46,XX,t(9;22)(q34;q11.2)[6]/46,XX,ider(22)(q10)t(9;22)(q34;q11.2)[14]. Fluorescence in situ hybridization (FISH) analysis for BCR::ABL1 fusion showed 40%, of interphase cells with two and 35%, with three fusion signals that were in concordance with the karyotype. The patient was categorized as National Cancer Institute (NCI) high-risk (HR) and started with HR chemotherapy according to Children's Oncology Group (COG) protocol. Postinduction remission assessment by flow cytometry showed 2.6%, measurable residual disease. The case highlights significance of cytogenetic analysis despite availability of advanced techniques like FISH. The prognostic significance of concurrent ider22(q10) with t(9;22) is yet to be explored.
Keywords
acute lymphoblastic leukemia - immunophenotyping - Philadelphia chromosome - cytogenetic analysis - measurable residual diseaseAuthors' Contributions
Neelum Mansoor is involved in the study of concept and design. Syeda Ambareen Zehra did the acquisition of data. Sidra Maqsood drafted the manuscript. Imad Bakri was involved in the critical revision of the manuscript for important intellectual content. Sidra Maqsood and Syeda Ambareen Zehra are involved in administrative, technical, and material support. Neelum Mansoor supervised the study.
Patient Consent
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Publication History
Article published online:
05 July 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Rare Association of Tuberous sclerosis with Acute Lymphoblastic Leukemia: Case Report with Review of LiteratureAbhilasha Sampagar, Indian Journal of Medical and Paediatric Oncology
- Multiple Complications Secondary to L-asparaginase In a Child with Philadelphia-Chromosome-Positive Acute Lymphoblastic Leukemia: Case Report with Review of Lit...Shyam Srinivasan, VCOT Open, 2022
- Multiple Complications Secondary to L-asparaginase In a Child with Philadelphia-Chromosome-Positive Acute Lymphoblastic Leukemia: Case Report with Review of Lit...Shyam Srinivasan, Indian Journal of Medical and Paediatric Oncology, 2022
- Rare Association of Tuberous sclerosis with Acute Lymphoblastic Leukemia: Case Report with Review of LiteratureAbhilasha Sampagar, VCOT Open
- Isolated Wrist Drop Presenting as Acute Stroke: Rare Case Report with Review of LiteratureKhushbu Goel, Journal of Neurosciences in Rural Practice, 2018
- Spontaneous Coronary Artery Dissection – Case Report and Literature ReviewElana Couto de Alencar Daniel, Arquivos Brasileiros de Cardiologia, 2019
- Carcinoid Heart Disease: A Case Report and Literature ReviewIsabela Bispo Santos da Silva Costa, Arquivos Brasileiros de Cardiologia, 2023
- Eosinophilic Myocarditis: Clinical Case and Literature ReviewPaulo Dinis, Arquivos Brasileiros de Cardiologia, 2018
- Sternal stress fracture in a gymnast : a case report and literature review : case studyIsmail Hassan, South African Journal of Sports Medicine, 2010
- Recurrent Atrial Myxoma in a Patient with Carney Complex. A Case Report and Literature ReviewLaura A. Cervantes-Molina, Arquivos Brasileiros de Cardiologia, 2020
Abstract
The Philadelphia chromosome is seen in 5%, of pediatric and 25 to 50%, of adult cases of acute lymphoblastic leukemia (ALL). It is linked to aggressive illness with a dismal prognosis. Additional chromosomal abnormalities are not prevalent with translocation 9;22; nevertheless, isochromosome derivative [ider(22)] with this translocation is rarely recorded in the literature. This is the third instance of ider(22) in pediatric B-cell acute lymphoblastic leukemia (B-ALL). Bone marrow chromosome analysis by G-banding showed 46,XX,t(9;22)(q34;q11.2)[6]/46,XX,ider(22)(q10)t(9;22)(q34;q11.2)[14]. Fluorescence in situ hybridization (FISH) analysis for BCR::ABL1 fusion showed 40%, of interphase cells with two and 35%, with three fusion signals that were in concordance with the karyotype. The patient was categorized as National Cancer Institute (NCI) high-risk (HR) and started with HR chemotherapy according to Children's Oncology Group (COG) protocol. Postinduction remission assessment by flow cytometry showed 2.6%, measurable residual disease. The case highlights significance of cytogenetic analysis despite availability of advanced techniques like FISH. The prognostic significance of concurrent ider22(q10) with t(9;22) is yet to be explored.
Keywords
acute lymphoblastic leukemia - immunophenotyping - Philadelphia chromosome - cytogenetic analysis - measurable residual diseaseIntroduction
Philadelphia (Ph) chromosome is less often found in B-cell acute lymphoblastic leukemia (B-ALL) patients; however, it is commonly (90–95%) present in chronic myeloid leukemia (CML) patients.[1] [2] Ph chromosome can be detected in about 25%, of adult ALL and only 2 to 4%, of pediatric ALL.[1] [3] Presence of double Ph chromosome is infrequent in ALL but reported in some cases of CML during the blast crisis phase.[4] When compared to Ph chromosome-negative ALL, Ph chromosome-positive ALL is typically associated with a more aggressive disease that may be more resistant to treatment and can have a poorer prognosis compared to other types of pediatric ALL.[1] [5] Currently, the mainstay of treatment is a tyrosine kinase inhibitor plus intensive chemotherapy, followed by hematopoietic stem cell transplant (HSCT) after the first remission.[6] We report a rare case of a 4-year-old girl diagnosed as a case of isoderivative Ph chromosome-positive B-ALL. This chromosomal aberration is exceptionally rare in B-ALL. The case is being reported to spread awareness about significant additional cytogenetic findings in a case of B-ALL with recurrent cytogenetic abnormalities. This patient also revealed other poor clinical features like hyperleukocytosis at the time of presentation and did not achieve postinduction remission.
Case Report
A 4-year-old girl presented to emergency department of pediatric oncology unit with a history of recurrent epistaxis for 2 months and was severely anemic. There is no significant history of any past illness and/or genetic disease in the family. On general physical examination, she had lymphadenopathy and hepatosplenomegaly.
Differential Diagnosis, Investigations, and Treatment
Complete blood count showed hyperleukocytosis with white cell count of 153 × 109/L, anemia with hemoglobin of 7.5 gm/dL, and thrombocytopenia with a platelet count of 18 × 109/L. Blood film showed a leucoerythroblastic picture with 84%, blasts. Immunophenotyping performed on peripheral blood using 8-color flow cytometry (BD FACS Canto II) revealed the following immunophenotypic profile:
Positive for CD34, CD45, CD19, CD79a, CD20, CD10, CD66, CD9 and CD58
Negative for intra-cytoplasmic CD3, intra-cytoplasmic MPO, CD13, CD33
On the basis of flowcytometry, the case was diagnosed as B-ALL ([Fig. 1]). Other laboratory investigations including liver and kidney function tests were within normal range; however, serum albumin was low (2.0 g/dL). The diagnostic lumbar puncture for central nervous system (CNS) infiltration showed CNS1 status that is consistent with absence of blasts. Bone marrow specimen was received for fluorescence in situ hybridization (FISH) and cytogenetic analysis by G-banding ([Fig. 2]). Interphase FISH revealed atypical signal pattern, comprised of three fusion signals that were further evaluated by metaphase FISH ([Fig. 2B–D]). FISH analysis of the bone marrow for BCR::ABL1 dual color dual fusion probe using Leica Biosystems automated cell imaging system (CytoVision) detected 70%, of BCR::ABL1 fusion with 35%, of those cells harboring three fusion signals, indicating the presence of extra Ph chromosome. Karyotype analysis revealed the presence of an abnormal female chromosome complement comprised of two related cell lines. The first cell line (stem line) was seen in six cells with a Ph chromosome derived by a balanced translocation between the long arms of chromosomes 9 and 22. The second cell line (side line) is seen in 14 cells with an isochromosome for the long arm of chromosome 22 resulting by t(9;22; [Fig. 2A]) These findings were consistent with FISH results. Translocation (9;22) results in the fusion of the ABL1 gene at 9q34 and the BCR gene at 22 q11.2 that is associated with poor prognosis in B-ALL. The presence of ider(22) is very uncommon in pediatric B-ALL. She was categorized as NCI high-risk and received modified COG protocol along with Tyrosine kinase inhibitor (TKI).
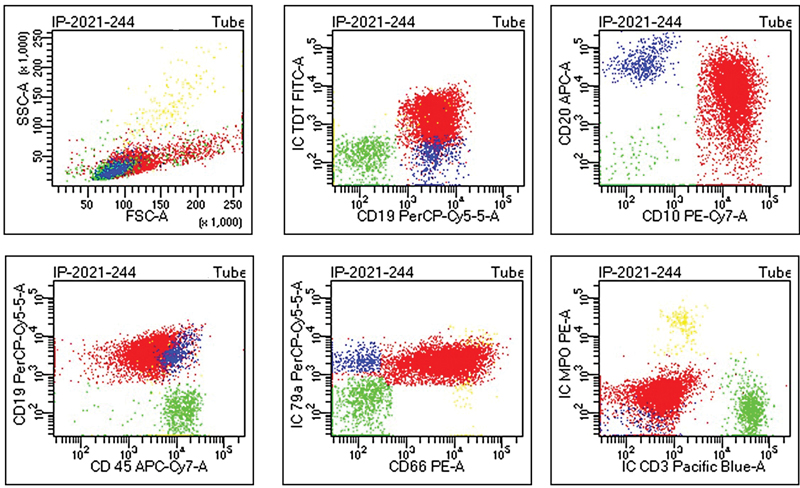
| | Fig 1Flow cytometry showing positivity for Tdt, CD19, CD10, CD20, CD45, CD79a, CD66 (color code: red = blasts, blue = B-lymphocytes, green =T-lymphocytes, yellow = granulocytes).|
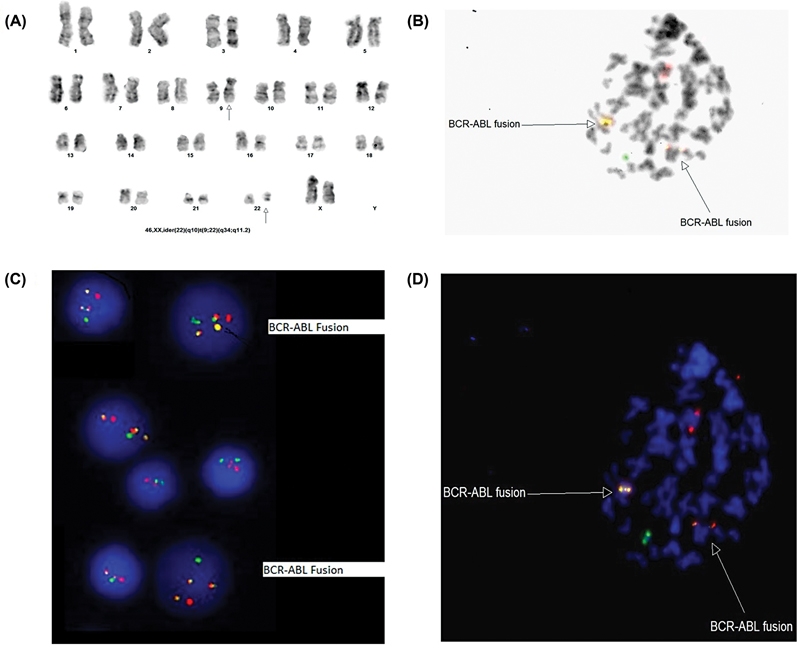
| Fig 2 (A) Karyotype, (B) met FISH with inverted 4'6-Diamidino-2-phenylindole (DAPI), and (C) interphase fluorescence in situ hybridization (FISH), (D) met FISH.|
Outcome and Follow-Up
Postinduction bone marrow aspirate on morphological review showed remission; however, measurable residual disease assessment by flowcytometry showed 2.6%, residual disease. A HSCT was not possible due to financial constraints; therefore, chemotherapy was adjusted accordingly. Currently, she is on high-risk consolidation chemotherapy.
We have summarized the features of our study case and other published cases in [Table 1.]
|
Studies |
Age/ Sex |
Clinical features |
G-banding or FISH Analysis |
Treatment |
Outcome |
|---|---|---|---|---|---|
|
Ramachandran et al (2016)[16] Patient 1 |
29y/M |
Massive splenomegaly |
46,XY,der(9)t(9;22)(q34.13;q11.23),ider(22) (p12)t(9;22)[7]/47,sl, + ider(22)[21]/48,sdl, + ider(22)[2] |
IM 400 mg daily (8.5 years) |
BM study revealed 8%, blasts, suggestive of CML-CP. The patient was started on second line TKI, dasatinib, and attained hematological remission 1 month later; now he is on dasatinib for 2 months |
|
Patient 2 |
51y/M |
Moderate splenomegaly |
46,XY,t(9;22)(q34.13;q11.23)[4]/46,–der(22)t(9;22), +ider(22)(p12)t(9;22)[4]/47,sdl1, + ider(22)[13]/48,sl, +der(22), + ider(22)[4]/47,sl, + 8[5] |
IM 400 mg daily (6 months) |
Initially responded, but subsequently lost the hematologic response. Switch to second line TKI, dasatinib |
|
Meza-Espinoza et al (2016) |
54y/F |
– |
46,XX,t(9;22) (q34;q11.2)[3]/46,idem,ider(22)(22pter→22q11.2::9q34→9q? tel::9q?tel→9q34::22q11.2→22pter)[14]/46,idem,t(13;17) (q14;q25)[3]/46,idem, + 1,dic(1;1)(?;?),t(13;17)(q14;q25) [8]/46,XX[1] |
Received vincristine, prednisone, and daunorubicin-based chemotherapy |
04 months later, the patient died after infiltrations were detected both to the retro-ocular and central nervous systems |
|
Our study |
4y/F |
Lymphadenopathy and hepatosplenomegaly |
46,XX,ider(22)(q10)t(9;22)(q34;q11.2) |
She was categorized as NCI high-risk and received modified COG protocol along with imatinib |
Postinduction bone marrow aspirate on morphological review showed remission however measurable residual disease (MRD) assessment by flowcytometry showed 2.6%, residual disease |
References
- Kang ZJ, Liu YF, Xu LZ. et al. The Philadelphia chromosome in leukemogenesis. Chin J Cancer 2016; 35 (01) 48
- Koo HH. Philadelphia chromosome-positive acute lymphoblastic leukemia in childhood. Korean J Pediatr 2011; 54 (03) 106-110
- Tang YL, Raja Sabudin RZ, Leong CF. et al. Double Philadelphia chromosome-positive B acute lymphoblastic leukaemia in an elderly patient. Malays J Pathol 2015; 37 (03) 275-279
- Siddappa S, Hassan SA, Lingappa KB. et al. Double Philadelphia chromosomes- a rare, yet an important cytogenetic phenomenon of prognostic significance in de novo acute lymphoblastic leukemia. Indian J Hematol Blood Transfus 2022; 38 (04) 739-744
- Rowe JM, Buck G, Burnett AK. et al; ECOG, MRC/NCRI Adult Leukemia Working Party. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood 2005; 106 (12) 3760-3767
- Ottmann OG, Wassmann B. Treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematology (Am Soc Hematol Educ Program) 2005; 2005 (01) 118-122
- Forestier E, Johansson B, Gustafsson G. et al; For the Nordic Society of Paediatric Haematology and Oncology (NOPHO) Leukaemia Cytogenetic Study Group. Prognostic impact of karyotypic findings in childhood acute lymphoblastic leukaemia: a Nordic series comparing two treatment periods. Br J Haematol 2000; 110 (01) 147-153
- Nordgren A, Heyman M, Sahlén S. et al. Spectral karyotyping and interphase FISH reveal abnormalities not detected by conventional G-banding. Implications for treatment stratification of childhood acute lymphoblastic leukaemia: detailed analysis of 70 cases. Eur J Haematol 2002; 68 (01) 31-41
- Achandira UM, Pathare AV, Kindi SA, Dennison D, Yahyaee SA. Isochromosome 9q as a sole anomaly in an Omani boy with acute lymphoblastic leukaemia. BMJ Case Rep 2009; 2009: bcr09.2008.0890
- Huh J, Chung W. [A case of acute lymphoblastic leukemia with ider(9)(q10)t(9;22)(q34;q11.2).]. Korean J Lab Med 2006; 26 (03) 223-226
- Kansal R, Deeb G, Barcos M. et al. Precursor B lymphoblastic leukemia with surface light chain immunoglobulin restriction: a report of 15 patients. Am J Clin Pathol 2004; 121 (04) 512-525
- Preiss BS, Kerndrup GB, Pedersen RK, Hasle H, Pallisgaard N. Lymphoma-Leukemia Study Group of the Region of Southern Denmark. Contribution of multiparameter genetic analysis to the detection of genetic alterations in hematologic neoplasia. An evaluation of combining G-band analysis, spectral karyotyping, and multiplex reverse-transcription polymerase chain reaction (multiplex RT-PCR). Cancer Genet Cytogenet 2006; 165 (01) 1-8
- Yamamoto K, Nagata K, Morita Y, Inagaki K, Hamaguchi H. Isodicentric Philadelphia chromosome in acute lymphoblastic leukemia with der(7;12)(q10;q10). Leuk Res 2007; 31 (05) 713-718
- Meza-Espinoza JP, Romo Martínez EJ, Aguilar López L, Picos Cárdenas VJ, Magaña Torres MT, González García JR. B-cell acute lymphoblastic leukemia with t(9;22)(q34;q11) translocation and clonal divergence through ider(22) chromosome and t(13;17)(q14;q25) translocation. Ann Lab Med 2016; 36 (02) 185-187
- Mitelman F, Johansson B, Mertens F. Eds. Mitelman database of chromosome aberrations and gene fusions in cancer. (Updated on Nov 19, 2014). Accessed May 31, 2023 at: http://cgap.nci.nih.gov/Chromosomes/Mitelman
-
Ramachandran KC,
Narayanan G,
Nair SG,
Thambi SM,
Kamala LH,
Gopinath P,
Sreedharan H.
Isodicentric Philadelphia Chromosome: A Rare Chromosomal Aberration in Imatinib-Resistant Chronic Myelogenous Leukemia Patients-Case Report with Review of the Literature. Cytogenetic and Genome Research 2016; Jan 1;150(3-4): 273-280
Address for correspondence
Neelum Mansoor, FCPSConsultant Hematologist, Indus Hospital & Health NetworkKorangi Campus, Plot C-76, Sector 31/5, Opposite Darussalam Society Korangi Crossing, Karachi -75190PakistanEmail: neelum.mansoor@tih.org.pkPublication History
Article published online:
05 July 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, IndiaWe recommend- Rare Association of Tuberous sclerosis with Acute Lymphoblastic Leukemia: Case Report with Review of LiteratureAbhilasha Sampagar, Indian Journal of Medical and Paediatric Oncology
- Multiple Complications Secondary to L-asparaginase In a Child with Philadelphia-Chromosome-Positive Acute Lymphoblastic Leukemia: Case Report with Review of Lit...Shyam Srinivasan, VCOT Open, 2022
- Multiple Complications Secondary to L-asparaginase In a Child with Philadelphia-Chromosome-Positive Acute Lymphoblastic Leukemia: Case Report with Review of Lit...Shyam Srinivasan, Indian Journal of Medical and Paediatric Oncology, 2022
- Rare Association of Tuberous sclerosis with Acute Lymphoblastic Leukemia: Case Report with Review of LiteratureAbhilasha Sampagar, VCOT Open
- Isolated Wrist Drop Presenting as Acute Stroke: Rare Case Report with Review of LiteratureKhushbu Goel, Journal of Neurosciences in Rural Practice, 2018
- Philadelphia chromosome-positive granular acute lymphoblastic leukemia: report of one case and review of literatureCMA Publishing House, 2023
- Chronic myelogenous leukemia with positive Philadelphia chromosome and AML1-ETO fusion gene: report of one case and review of literatureCMA Publishing House, 2023
- Acute lymphoblastic leukemia combined with rhabdomyolysis: report of 1 case and review of literatureCMA Publishing House, 2023
- De novo Philadelphia chromosome-positive myelodysplastic syndrome: report of 1 case and review of literatureCMA Publishing House, 2023
- Prognostic analysis of children with Philadelphia chromosome-like acute lymphoblastic leukemia common genesCMA Publishing House, 2022
- Rare Association of Tuberous sclerosis with Acute Lymphoblastic Leukemia: Case Report with Review of Literature

| | Fig 1Flow cytometry showing positivity for Tdt, CD19, CD10, CD20, CD45, CD79a, CD66 (color code: red = blasts, blue = B-lymphocytes, green =T-lymphocytes, yellow = granulocytes).|

| Fig 2 (A) Karyotype, (B) met FISH with inverted 4'6-Diamidino-2-phenylindole (DAPI), and (C) interphase fluorescence in situ hybridization (FISH), (D) met FISH.|
References
- Kang ZJ, Liu YF, Xu LZ. et al. The Philadelphia chromosome in leukemogenesis. Chin J Cancer 2016; 35 (01) 48
- Koo HH. Philadelphia chromosome-positive acute lymphoblastic leukemia in childhood. Korean J Pediatr 2011; 54 (03) 106-110
- Tang YL, Raja Sabudin RZ, Leong CF. et al. Double Philadelphia chromosome-positive B acute lymphoblastic leukaemia in an elderly patient. Malays J Pathol 2015; 37 (03) 275-279
- Siddappa S, Hassan SA, Lingappa KB. et al. Double Philadelphia chromosomes- a rare, yet an important cytogenetic phenomenon of prognostic significance in de novo acute lymphoblastic leukemia. Indian J Hematol Blood Transfus 2022; 38 (04) 739-744
- Rowe JM, Buck G, Burnett AK. et al; ECOG, MRC/NCRI Adult Leukemia Working Party. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood 2005; 106 (12) 3760-3767
- Ottmann OG, Wassmann B. Treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematology (Am Soc Hematol Educ Program) 2005; 2005 (01) 118-122
- Forestier E, Johansson B, Gustafsson G. et al; For the Nordic Society of Paediatric Haematology and Oncology (NOPHO) Leukaemia Cytogenetic Study Group. Prognostic impact of karyotypic findings in childhood acute lymphoblastic leukaemia: a Nordic series comparing two treatment periods. Br J Haematol 2000; 110 (01) 147-153
- Nordgren A, Heyman M, Sahlén S. et al. Spectral karyotyping and interphase FISH reveal abnormalities not detected by conventional G-banding. Implications for treatment stratification of childhood acute lymphoblastic leukaemia: detailed analysis of 70 cases. Eur J Haematol 2002; 68 (01) 31-41
- Achandira UM, Pathare AV, Kindi SA, Dennison D, Yahyaee SA. Isochromosome 9q as a sole anomaly in an Omani boy with acute lymphoblastic leukaemia. BMJ Case Rep 2009; 2009: bcr09.2008.0890
- Huh J, Chung W. [A case of acute lymphoblastic leukemia with ider(9)(q10)t(9;22)(q34;q11.2).]. Korean J Lab Med 2006; 26 (03) 223-226
- Kansal R, Deeb G, Barcos M. et al. Precursor B lymphoblastic leukemia with surface light chain immunoglobulin restriction: a report of 15 patients. Am J Clin Pathol 2004; 121 (04) 512-525
- Preiss BS, Kerndrup GB, Pedersen RK, Hasle H, Pallisgaard N. Lymphoma-Leukemia Study Group of the Region of Southern Denmark. Contribution of multiparameter genetic analysis to the detection of genetic alterations in hematologic neoplasia. An evaluation of combining G-band analysis, spectral karyotyping, and multiplex reverse-transcription polymerase chain reaction (multiplex RT-PCR). Cancer Genet Cytogenet 2006; 165 (01) 1-8
- Yamamoto K, Nagata K, Morita Y, Inagaki K, Hamaguchi H. Isodicentric Philadelphia chromosome in acute lymphoblastic leukemia with der(7;12)(q10;q10). Leuk Res 2007; 31 (05) 713-718
- Meza-Espinoza JP, Romo Martínez EJ, Aguilar López L, Picos Cárdenas VJ, Magaña Torres MT, González García JR. B-cell acute lymphoblastic leukemia with t(9;22)(q34;q11) translocation and clonal divergence through ider(22) chromosome and t(13;17)(q14;q25) translocation. Ann Lab Med 2016; 36 (02) 185-187
- Mitelman F, Johansson B, Mertens F. Eds. Mitelman database of chromosome aberrations and gene fusions in cancer. (Updated on Nov 19, 2014). Accessed May 31, 2023 at: http://cgap.nci.nih.gov/Chromosomes/Mitelman
- Ramachandran KC, Narayanan G, Nair SG, Thambi SM, Kamala LH, Gopinath P, Sreedharan H. Isodicentric Philadelphia Chromosome: A Rare Chromosomal Aberration in Imatinib-Resistant Chronic Myelogenous Leukemia Patients-Case Report with Review of the Literature. Cytogenetic and Genome Research 2016; Jan 1;150(3-4): 273-280


 PDF
PDF  Views
Views  Share
Share

