Assessment of Cranial Radiotherapy Treatment in T-cell Lymphoblastic Lymphoma: Retrospective Study from Tertiary Care Center
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2022; 43(02): 177-184
DOI: DOI: 10.1055/s-0042-1743507
Abstract
Introduction Leukemia-like regimens given for acute lymphoblastic leukemia (ALL) are the cornerstone of treatment for T-cell lymphoblastic lymphoma (LBL) and can produce complete remission rates exceeding 90%. For central nervous system (CNS) prophylaxis, intrathecal chemotherapy and cranial irradiation are used to prevent future CNS recurrence.
Objective The purpose of this study was to assess CNS relapse rate after cranial prophylaxis treatment given at our institute.
Materials and Methods In this retrospective analysis, between July 2013 and June 2019, 149 files of lymphoblastic lymphoma were reviewed. Out of these, 53 patients received cranial irradiation. All patients were given CNS-directed therapy in the form of intrathecal methotrexate and patients with CNS-negative disease and primary tumor complete response or more than partial response after chemotherapy were given prophylactic cranial irradiation (18 Gy/10#), and in patients with upfront CNS involvement, therapeutic cranial irradiation (24 Gy/12#) was delivered. Radiotherapy was delivered as per the standard conventional protocol on a linear accelerator.
Results Out of 53 patients (age range: 2–50 years, mean–16.79 years, 26 [49.1%] pediatric [<14>14 years]), 13/53 (24.5%), and 40/53 (75.5%) patients were on MCP 841 and BFM 90 protocols, respectively. Also, 48 (90.56%) patients received prophylactic cranial irradiation (25 [52.1%] pediatric, 23 [47.9%] adults). Moreover, 3/48 (6.25%) (2 [4.16%] pediatric, 1 [2.08%] adult) patients had CNS failure after receiving prophylactic cranial irradiation. For 48 target patients, with the median follow-up of 27.27 months (26.1 months–pediatric, 28.2 months– adults), EFS (event-free survival) in the brain was 93.8% (92%: pediatric, 95.7%: adults). Also, the difference between pediatric and adult groups was not statistically significant (p-value = 0.662). Five (9.43%) patients had CNS-positive disease upfront and received therapeutic cranial irradiation.
Conclusion In BFM 90/MCP 841 protocol in lymphoblastic lymphoma, prophylactic cranial irradiation and intrathecal methotrexate have been the standard of care as the CNS-directed therapy to prevent cranial infiltration. Though our results are not at par with the published world literature, further research and efforts are required to prevent CNS relapse in a selected sub-set of patients with lymphoblastic lymphoma.
Keywords
CNS relapse - cranial radiotherapy - lymphoblastic lymphoma
Authors' Contributors
Dr. Maitrik Mehta proposed the stated study objective to be assessed and contributed to data collection, analysis of the data, conception and design, drafting the article, and final approval of the version to be published. Dr. Isha Shah helped in collecting and analyzing the data and helped in the preparation of the manuscript. Dr. Harsha Panchal helped in manuscript assessment and analysis of data. Dr. Ankita Parikh, Dr. U. Suryanarayan, Dr. Jayesh Singh, Dr. Arun T. helped in manuscript editing. All authors approved the final version of the manuscript.
Publication History
Article published online:
29 April 2022
© 2022. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction Leukemia-like regimens given for acute lymphoblastic leukemia (ALL) are the cornerstone of treatment for T-cell lymphoblastic lymphoma (LBL) and can produce complete remission rates exceeding 90%. For central nervous system (CNS) prophylaxis, intrathecal chemotherapy and cranial irradiation are used to prevent future CNS recurrence.
Objective The purpose of this study was to assess CNS relapse rate after cranial prophylaxis treatment given at our institute.
Materials and Methods In this retrospective analysis, between July 2013 and June 2019, 149 files of lymphoblastic lymphoma were reviewed. Out of these, 53 patients received cranial irradiation. All patients were given CNS-directed therapy in the form of intrathecal methotrexate and patients with CNS-negative disease and primary tumor complete response or more than partial response after chemotherapy were given prophylactic cranial irradiation (18 Gy/10#), and in patients with upfront CNS involvement, therapeutic cranial irradiation (24 Gy/12#) was delivered. Radiotherapy was delivered as per the standard conventional protocol on a linear accelerator.
Results Out of 53 patients (age range: 2–50 years, mean–16.79 years, 26 [49.1%] pediatric [<14>14 years]), 13/53 (24.5%), and 40/53 (75.5%) patients were on MCP 841 and BFM 90 protocols, respectively. Also, 48 (90.56%) patients received prophylactic cranial irradiation (25 [52.1%] pediatric, 23 [47.9%] adults). Moreover, 3/48 (6.25%) (2 [4.16%] pediatric, 1 [2.08%] adult) patients had CNS failure after receiving prophylactic cranial irradiation. For 48 target patients, with the median follow-up of 27.27 months (26.1 months–pediatric, 28.2 months– adults), EFS (event-free survival) in the brain was 93.8% (92%: pediatric, 95.7%: adults). Also, the difference between pediatric and adult groups was not statistically significant (p-value = 0.662). Five (9.43%) patients had CNS-positive disease upfront and received therapeutic cranial irradiation.
Conclusion In BFM 90/MCP 841 protocol in lymphoblastic lymphoma, prophylactic cranial irradiation and intrathecal methotrexate have been the standard of care as the CNS-directed therapy to prevent cranial infiltration. Though our results are not at par with the published world literature, further research and efforts are required to prevent CNS relapse in a selected sub-set of patients with lymphoblastic lymphoma.
Introduction
Lymphoblastic lymphoma (LBL) is a neoplasm of lymphoblasts, more commonly of T-cell origin than of B-cell origin that resembles acute lymphoblastic leukemia (ALL), as suggested by the 2008 World Health Organization classification.[1] [2] Usually, in most cases, bulky mediastinal disease in young men is the standard presentation of lymphoblastic lymphoma (the male-to-female ratio is 2:1). Leukemia-like regimens given for ALL are the cornerstone of treatment for T-cell lymphoblastic lymphoma and can produce complete remission rates exceeding 90%.[3] [4] The central nervous system (CNS) is involved at diagnosis in only 2 to 7%-of cases.[5] However, CNS relapse can be seen in up to one-third of LBL patients if CNS prophylaxis treatment is not delivered.[6] For CNS prophylaxis, intrathecal chemotherapy and cranial irradiation are used to prevent future CNS recurrence. Long-term toxicities of central nervous irradiation are great and not well studied in LBL.[7] The purpose of this study was to assess CNS relapse rate after cranial prophylaxis treatment given at our institute.
Materials and Methods
In this retrospective analysis, we assessed both pediatric and adult patients with lymphoblastic lymphoma registered in our institute between July 2013 and June 2019. We retrieved files from the medical record department with permission. A total of 149 patients were registered during this period. Out of 149, 53 patients had received cranial irradiation and were assessed for the stated objective.
For all patients (pediatric and adults) who had biopsy-proven T-cell lymphoblastic lymphoma and received cranial irradiation (prophylactic/therapeutic), we retrieved data including clinical presentation, investigations done, chemotherapy protocol used, cranial radiotherapy planning (basic details of planning mentioned in files as per the department protocol) and last follow-up with clinical status.
Treatment protocol choice was dependent on consultant decision, patients' general condition, age, government beneficiary scheme, and financial status of the patient.
-
MCP 841 protocol,[8] included induction, induction phase II with cranial irradiation, reinduction, consolidation, and maintenance for 2 years.
-
BFM 90 (Berlin Frankfurt Munster) protocol[9] [10] included preinduction, induction, and consolidation, reinduction chemotherapy followed by cranial irradiation, and maintenance chemo for up to 2 years.
-
Mostly, pediatric and adolescent patients received the BFM 90 protocol treatment and adult patients received the MCP 841 protocol treatment.
Response rates following chemotherapy (after induction in MCP 841 and after reinduction in BFM 90) were assessed by objective assessment (in most of the cases, CT scans). Patients with complete or partial response were referred for prophylactic cranial irradiation.
CNS-directed cranial prophylaxis treatment in the form of intrathecal methotrexate and prophylactic cranial irradiation was given in patients with CNS-negative disease, primary tumor complete response (CR), or partial response (PR) following chemotherapy.
Before starting prophylactic cranial irradiation, mandatory baseline investigations were CBC (Hb ≥ to 10 g%, ANC count > 1,000 cells/µL), bone marrow under remission (if involved), and CSF cytology-negative disease. We see for mediastinal mass to be under complete remission following chemotherapy, but, in a few subsets of patients, we looked for partial response (PR) following chemotherapy.
In patients with CSF cytology-positive disease or magnetic resonance imaging (MRI) suggestive of brain infiltration, we had given therapeutic cranial irradiation.
In all patients, whole-brain radiotherapy was given by slanting field technique ([Fig. 1]).
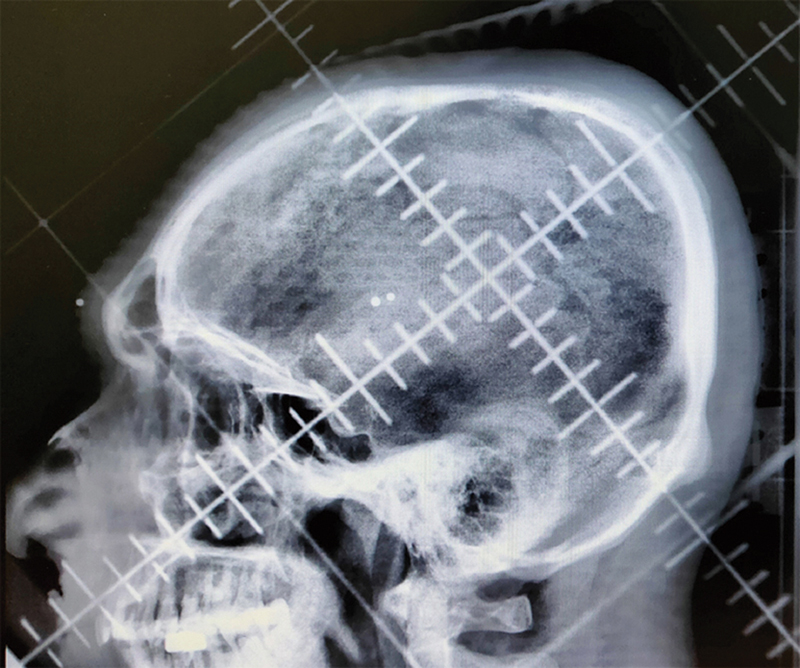
| Fig. 1Slanting filed radiotherapy planning skiagram showing field borders.
German helmet or slanting field technique is the most commonly used technique for whole-brain radiation therapy in developing countries. These techniques are simple, easy to perform, and are of low cost, making them widely popular in busy centers with limited facilities.
In all patients, marking was done on a conventional simulator.
For pediatric patients, a special immobilization device was developed in our radiotherapy department ([Fig. 2]).
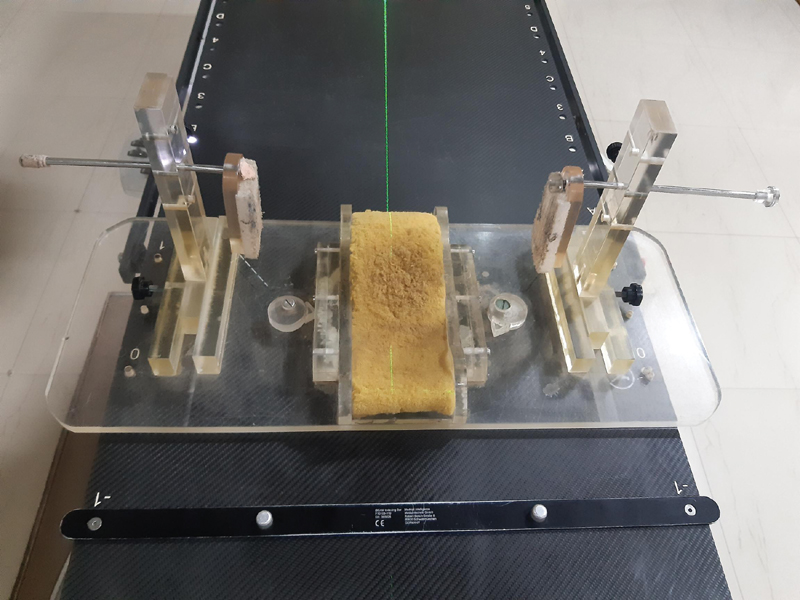
| Fig.2:Special immobilization device developed at our institute for pediatric patients. Mainly used to fix head position.
For adults, we asked patients to lie supine on the treatment couch with hands by the side and neck fully extended with the head resting in place during treatment.
For the radiotherapy treatment field, the superior border is just above the superior aspect of the skull with a minimum of 1 cm coverage, the inferior border is through the frontal sinus extending to cover the bones of the base of the skull. It also includes the spinous process of C1 and through the spinous process of C2 to ensure the posterior fossa is covered. The anterior border is anterior to the anterior aspect of the skull with a minimum of 1 cm coverage and the posterior border is posterior to the posterior aspect of the skull with a minimum of 1 cm coverage.
Technique: Bilateral conventional portals (slanting field technique)
Prophylactic cranial radiotherapy
Dose: 18 Gy/10#/5 days a week/2 weeks.
Therapeutic cranial irradiation
Dose: 24 Gy/12#/5 days a week/3 weeks.
We did not give craniospinal irradiation for CNS-positive disease.
For patients who had received prophylactic cranial irradiation, the respective medical department had advised for CSF cytology during follow-up assessment as per the symptoms.
The primary endpoint of the study was to assess CNS relapse rate after cranial prophylaxis treatment given at our institute in T-cell lymphoblastic lymphoma patients.
The primary aim of this study was to assess CNS relapse rate after cranial prophylaxis treatment given at our institute. So, only patients allocated to the target group–48 patients (CNS negative, and patients with CR or PR) were included and we calculated the event-free survival (EFS). EFS was calculated from the date of diagnosis to the event (CNS relapse). We excluded five patients who presented with upfront CNS involvement. Survival functions were calculated according to the Kaplan–Meier method,[11] with differences compared (between pediatric and adults) using the log-rank test.[12] Patients lost to follow-up were censored at the time of their last follow-up examination.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Science (SPSS) software version 21 (IBM SPSS Statistics for Windows; Armonk, New York, United States; IBM Corp.). Continuous variables are reported as median with standard deviation (SD) and categorical variables as percentages. The association between categorical variables was evaluated for significance, period of treatment to relapse was calculated in each risk group, and Student's t-test was applied to study the relationship between relapse rates of the pediatric and adult populations. The test was considered significant if the p-value was < 0.05.
Ethics
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964, as revised in 2013. The Gujarat Cancer & Research Institute (GCRI) Ethics Committee board approval was obtained (No: IRC/2021/P-58 on August 24, 2021). Because the study was retrospective, informed consent was not required and the study did not include any intervention. Waiver of informed consent was obtained from the Ethics Committee.
Results
In this retrospective analysis, from July 2013 to June 2019, 149 patients (pediatric and adults) with newly diagnosed T-cell lymphoblastic lymphoma were reviewed. Of these, 54 (age range: 2–50 years) patients received cranial irradiation. One patient received prophylactic cranial irradiation and did not return for a follow-up. So, 53 patients who received cranial irradiation were assessed.
[Table 1] shows the characteristics of patients who received cranial irradiation in T-cell lymphoblastic lymphoma.
|
Characteristics |
Pediatric |
Adult |
Combined |
|---|---|---|---|
|
Age group |
<14> |
>14 years |
|
|
26 |
27 |
53 |
|
|
Mean |
9.65 years |
23.67 years |
16.79 years |
|
Median |
10 years |
22 years |
15 years |
|
Range |
2–13 years |
15–50 years |
2–50 years |
|
Sex |
|||
|
Male |
23 |
24 |
47/53 (88.6%) |
|
Female |
3 |
3 |
6/53 (11.3%) |
|
Stage |
Ann Arbor[24] |
||
|
Stage II |
3 |
13 |
16 (30.2%) |
|
Stage III |
14 |
– |
14 (26.4%) |
|
Stage IV |
9 |
14 |
23 (43.4%) |
|
CNS-negative disease upfront |
25 |
23 |
48 (90.56%) |
|
Bone marrow Involvement |
9 |
9 |
18 (33.9%) |
|
Mediastinal tumor |
17 |
13 |
30 (56.6%) |
|
CNS involvement upfront |
1 |
4 |
5 (9.43%) |
|
Treatment protocol |
|||
|
BFM 90 |
20/26 |
20/27 |
40/53 (75.5%) |
|
MCP 841 |
6/26 |
7/27 |
13/53 (24.5%) |
|
Characteristics |
Number of patients |
||
|---|---|---|---|
|
Pediatric |
Adult |
Combined |
|
|
1) CNS-negative disease |
25 |
23 |
48 (90.56%) |
|
Primary tumor |
|||
|
Complete response (CR) |
17/48 (35.4%) |
20/48 (41.7%) |
37/48 (77.08%) |
|
Partial response (PR) |
8/48 (16.7%) |
3/48 (6.25%) |
11/48 (22.9%) |
|
Radiotherapy |
|||
|
Prophylactic cranial irradiation received |
25/48 (52.1%) |
23/48 (47.9%) |
48 |
|
CNS failure after prophylactic cranial irradiation |
2/48 (4.16%) |
1/48 (2.08%) |
3/48 (6.25%) |
|
CNS failure after prophylactic cranial irradiation (in correlation to primary tumor response) |
|||
|
- Primary tumor CR |
0/17 |
1/20 |
1/37 |
|
- Primary tumor PR |
2/8 |
0/3 |
2/11 |
|
2) CNS-positive disease upfront |
|||
|
Therapeutic cranial irradiation |
1 |
4 |
5 (9.43%) |
|
Site of tumor failure (after PCI) |
|||
|
CNS (isolated) |
2 |
1 |
3 |
|
Testis |
1 |
– |
1 |
|
Testis + medullary |
1 |
2 |
2 |
|
Medullary |
1 |
1 |
3 |
|
Kidney |
– |
1 |
1 |
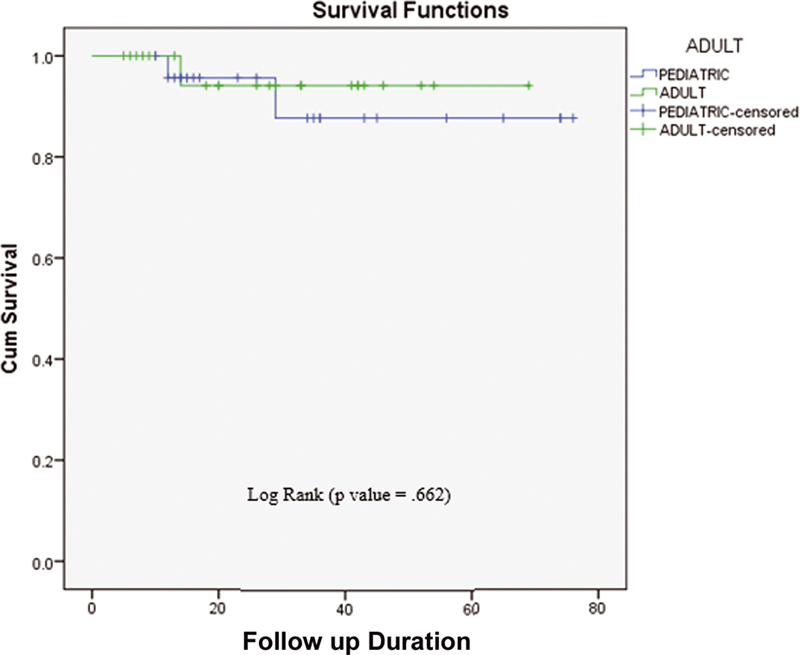
| Fig.3:Duration of Event Free Survival (EFS) in pediatric and adult patients.
Therapeutic Cranial Irradiation
Five patients (9.43%) (1 pediatric, 4 adults) had upfront CNS involvement and they received therapeutic cranial irradiation.
Discussion
Leukemia-like regimens given for ALL are the cornerstone of treatment for T-cell lymphoblastic lymphoma and can produce complete remission rates exceeding 90%.[3] [4] Central nervous system (CNS) is involved at diagnosis in only 2 to 7%-of cases.[5] However, CNS relapse can be seen in up to one-third of LBL patients if CNS prophylaxis treatment is not delivered.[6]
CNS relapse is mainly the dominant event once hematologic remission extends beyond 6 to 12 months.[13] So, CNS preventive therapy evolved through subsequent studies at St. Jude Children's Research Hospital (SJCRH) and in Cancer and Leukemia Group B. Craniospinal irradiation or cranial irradiation (CrI) at 24 Gy (CrI with intrathecal methotrexate (IT-MTX)), reduced the incidence of CNS relapse from >60%-to < 5 to 10%. Subsequent trials proved the advantage of CrI at 18 Gy combined with IT-MTX (intrathecal methotrexate) as the “standard” preventive regimen.[14] [15] [16]
The St. Jude study XI for childhood ALL has reported an isolated CNS relapse rate of 5%-[17] and Gelber et al[18] in their analysis have reported a rate of 6%.
In a study by Raje et al (MCP 841 protocol), out of 623 patients, 568 patients received cranial radiotherapy and 12 doses of intrathecal methotrexate, 10 (1.75%) patients had developed isolated CNS relapse.[8] In the MCP 841 protocol, the mainly pediatric patient population was included with age 3 to 6 years both inclusive and no mediastinal involvement. The cranial radiotherapy dose used was 20 Gy/10#. Out of the subjects who received cranial radiotherapy and 12 doses of intrathecal methotrexate, 10 patients had an isolated CNS relapse indicating a relapse rate of 1.76%. In addition, eight patients had CNS and bone marrow relapses. So, 18/568 (3.12%) patients had a CNS relapse rate.
In an initial study by Coleman et al[19] using an ALL protocol for lymphoblastic lymphoma patients, intrathecal therapy was not given initially until 8 weeks into therapy, and 5 of 14 patients had a relapse in the CNS before receiving therapy. When IT chemotherapy was administered earlier during the treatment, CNS relapse was far less common (1/30 patients).
In the Berlin–Frankfurt–Munster (BFM) trials on childhood NHL, patients with T-LBL were treated according to the strategy for acute lymphoblastic leukemia (ALL)[9] [10] (NHL-BFM 90). The patient population selected was also the pediatric group with an age range between 1.1 and 16.4 years. Preventive CNS therapy consisted of cranial radiation therapy (CRT), intrathecal methotrexate (MTX), and intravenous MTX. The dose of prophylactic CRT was reduced from 18 Gy to 12 Gy, and high-dose MTX 5 g/m2 was introduced. Preventive CNS therapy based on steroids, intrathecal MTX, intravenous high-dose MTX, and moderate-dose prophylactic CRT (12 Gy) proved highly efficient. Out of 105 patients, 1 patient had BM and CNS relapse combined with local tumor progression.
Our study was retrospective in nature. We have included both pediatric and adult age group patients. In addition, 88.6%-of patients were male. In our study, 75.5%-of patients were treated using the BFM 90 protocol and 24.5%-of patients were treated using the MCP 841 protocol. So, not all patients were uniformly randomized or treated using the same protocol. Also, we can see that 11/48 (22.9%) patients had partial response following chemotherapy when they were referred for radiation.
We can also see that all patients had received two-dimensional (2D) conventional radiotherapy (slanting field technique). In MCP 841 (cranial RT dose: 20 Gy/10#) and BFM 90 (cranial RT dose: 12 Gy/6#) protocols, different radiotherapy regimens were used. However, trials have proved the advantage of cranial irradiation at 18 Gy combined with IT-MTX as the standard preventive regimens in leukemia patients.[14] [15] [16] And, in our department for prophylactic cranial irradiation, we used the same dose as 18 Gy to all patients.
In our study, 3/48 (6.25%) (2/48 [4.16%] pediatric, and 1/48 [2.08%] adult) patients had CNS failure after CNS prophylaxes treatment. As techniques have improved a lot and proper dose distribution can be achieved with 3DCRT/IMRT techniques, we can change the technique for prophylactic cranial irradiation to treat patients. Also, differences in chemotherapy of both studies would have also played a role in a higher rate of CNS recurrence. As in the BFM 90 original protocol, there was less CNS relapse after a moderate dose of RT in prophylactic cranial irradiation.
Also, our study had limitations such as uneven distribution of patients, combined group of pediatric and adults patients compliance to treatment, lost to follow-up, retrospective analysis.
Also, only cranial irradiation does not play a role to prevent CNS relapse after CNS prophylaxis treatment. It is mainly advantageous to prevent future CNS relapse. As mentioned, there would have been other factors also such as elevated LDH, high leukemic cell proliferation index, T-cell immunophenotype, white blood cell (WBC) count at baseline, baseline testicular involvement also plays a role; however, we have not assessed for the same. Our aim was mainly to study that even after prophylactic cranial irradiation, how many patients develop CNS relapse. So, we need to study in further detail the criteria mentioned above (high-end RT techniques with baseline laboratory and molecular level assessment and clinical presentation) to assess for CNS relapse.
With the recognition of adverse effects of radiotherapy, several patient characteristics such as high WBC count, T-ALL, very young age, and male sex have also been identified which are at an increased risk of CNS relapse.[20]
For 48 target patients, with a median follow-up of 27.27 months (26.1 months: pediatric, 28.2 months: adults), EFS in the brain was 93.8% (92%: pediatric, 95.7%: adults). Also, the study was not a comparison between the pediatric and adult groups; however, based on statistical analysis, for both groups, the p-value (0.662) was generated and it was not statistically significant.
[Table 3] shows a comparison of our study with various studies.
|
Study |
Protocol |
N |
Cranial radiotherapy (CNS-negative) |
Median follow-up |
CNS relapse rates |
|---|---|---|---|---|---|
|
Coleman et al[19] |
2 ALL protocols |
44 (lymphoblastic lymphoma) |
24 Gy |
26 months |
3% (after starting IT MTX earlier in second protocol) (combined) |
|
ALL type protocols |
105 (lymphoblastic lymphoma) |
12 Gy |
4.5 years |
0.9% |
|
|
MCP 841[8] |
ALL protocol |
568 (acute lymphoblastic leukemia) |
20 Gy |
21 months |
1.76% (isolated) |
|
Our study |
ALL protocols |
48 (lymphoblastic lymphoma: CNS-negative disease) |
18 Gy |
27.27 months |
6.25% (isolated) |

| Fig. 1Slanting filed radiotherapy planning skiagram showing field borders.

| Fig.2:Special immobilization device developed at our institute for pediatric patients. Mainly used to fix head position.

| Fig.3:Duration of Event Free Survival (EFS) in pediatric and adult patients.
References
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011; 117 (19) 5019-5032
- Soslow RA, Baergen RN, Warnke RA. B-lineage lymphoblastic lymphoma is a clinicopathologic entity distinct from other histologically similar aggressive lymphomas with blastic morphology. Cancer 1999; 85 (12) 2648-2654
- Kantarjian HM, O'Brien S, Smith TL. et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol 2000; 18 (03) 547-561
- Kantarjian HM, Thomas D, Ravandi F. et al. Defining the course and prognosis of adults with acute lymphocytic leukemia in first salvage after induction failure or short first remission duration. Cancer 2010; 116 (24) 5568-5574
- Cortelazzo S, Ponzoni M, Ferreri AJM, Hoelzer D. Lymphoblastic lymphoma. Crit Rev Oncol Hematol 2011; 79 (03) 330-343
- Sweetenham JW, Mead GM, Whitehouse JM. Adult lymphoblastic lymphoma: high incidence of central nervous system relapse in patients treated with the Stanford University protocol. Ann Oncol 1992; 3 (10) 839-841
- Kolotas C, Daniel M, Demetriou L. et al. Long-term effects on the intelligence of children treated for acute lymphoblastic leukemia. Cancer Invest 2001; 19 (06) 581-587
- Raje NS, Vaidya SJ, Kapoor G. et al. Low incidence of CNS relapse with cranial radiotherapy and intrathecal methotrexate in acute lymphoblastic leukemia. Indian Pediatr 1996; 33 (07) 556-560
- Reiter A, Schrappe M, Parwaresch R. et al. Non-Hodgkin's lymphomas of childhood and adolescence; results of a treatment stratified for biological subtypes and stage: a report of the BFM Group. J Clin Oncol 1995; 13 (02) 359-372
- Müller-Weihrich S, Henze G, Jobke A. et al. BFM study 1975/81 for treatment of non-Hodgkin lymphoma of high malignancy in children and adolescents [article in German]. Klin Padiatr 1982; 194 (04) 219-225
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457-481
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50 (03) 163-170
- Hustu HO, Aur RJ. Extramedullary leukaemia. Clin Haematol 1978; 7 (02) 313-337
- Schrappe M, Reiter A, Henze G. et al. Prevention of CNS recurrence in childhood ALL: results with reduced radiotherapy combined with CNS-directed chemotherapy in four consecutive ALL-BFM trials. Klin Padiatr 1998; 210 (04) 192-199
- Rivera GK, Raimondi SC, Hancock ML. et al. Improved outcome in childhood acute lymphoblastic leukaemia with reinforced early treatment and rotational combination chemotherapy. Lancet 1991; 337 (8733): 61-66
- Abromowitch M, Ochs J, Pui CH. et al. High-dose methotrexate improves clinical outcome in children with acute lymphoblastic leukemia: St. Jude Total Therapy Study X. Med Pediatr Oncol 1988; 16 (05) 297-303
- Rivera GK, Pui CH, Hancock ML. et al. Update of St Jude Study XI for childhood acute lymphoblastic leukemia. Leukemia 1992; 6 (Suppl. 02) 153-156
- Gelber RD, Sallan SE, Cohen HJ. et al. CNS relapse in leukemias. Cancer 1993; 72: 261-270
- Coleman CN, Picozzi Jr VJ, Cox RS. et al. Treatment of lymphoblastic lymphoma in adults. J Clin Oncol 1986; 4 (11) 1628-1637
- Komp DM, Fernandez CH, Falletta JM. et al. CNS prophylaxis in ALL. Cancer 1982; 50: 1031-1036
- Burkhardt B, Woessmann W, Zimmermann M. et al. Impact of cranial radiotherapy on central nervous system prophylaxis in children and adolescents with central nervous system-negative stage III or IV lymphoblastic lymphoma. J Clin Oncol 2006; 24 (03) 491-499
- Murphy SB, Fairclough DL, Hutchison RE, Berard CW. Non-Hodgkin's lymphomas of childhood: an analysis of the histology, staging, and response to treatment of 338 cases at a single institution. J Clin Oncol 1989; 7 (02) 186-193
- Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin's lymphomas: dissimilarities from lymphomas in adults. Semin Oncol 1980; 7 (03) 332-339
- Rosenberg SA. Validity of the Ann Arbor staging classification for the non-Hodgkin's lymphomas. Cancer Treat Rep 1977; 61 (06) 1023-1027


 PDF
PDF  Views
Views  Share
Share

