Arecanut as an emerging etiology of oral cancers in India
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(02): 71-79
DOI: DOI: 10.4103/0971-5851.99726
Abstract
Arecanut (AN) usage is widespread in Asian countries, especially India and Taiwan. The incidence of oral cancer is increasing day by day, but there is no exponential increase with tobacco usage. Especially in the country like Taiwan where betel quid mostly do not contain tobacco, AN can be correlated with the increased incidence of cancer. There are different studies in the literature about AN and oral cancer but none of them have concluded with the definite pathway for carcinogenesis. The present paper includes reviews of the literature for AN and oral cancer and summarizes the possible mechanisms associated with AN-induced carcinogenesis; and we have also tried to propose pathway of carcinogenesis.
Publication History
Article published online:
13 April 2022
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Arecanut (AN) usage is widespread in Asian countries, especially India and Taiwan. The incidence of oral cancer is increasing day by day, but there is no exponential increase with tobacco usage. Especially in the country like Taiwan where betel quid mostly do not contain tobacco, AN can be correlated with the increased incidence of cancer. There are different studies in the literature about AN and oral cancer but none of them have concluded with the definite pathway for carcinogenesis. The present paper includes reviews of the literature for AN and oral cancer and summarizes the possible mechanisms associated with AN-induced carcinogenesis; and we have also tried to propose pathway of carcinogenesis.
INTRODUCTION
Burden of oral malignant disease and premature death related to that is the burning issue in Asian countries. The association of betel quid with cancer could be concluded almost 100 years back; from the pre-Christian era, the records could be traced and was used as medical as well as psychosomatic substance as a breath refresher, digestive agent, worm expellant, aphrodisiac, and to maintain stamina.[1,2] In the recent era, the usage of betel quid was reintroduced almost 400 years when it was introduced from European traders.[1] The increased incidence of cancer in the recent population can be due to the change in the method of usage, i.e., keeping at particular site rather than rapid chewing and swallowing of all the contents, thereby decreasing direct contact time with the oral mucosa. The use of betel quid has become culturally accepted practice in India, which has now become a public health problem.[3,4]
The concept about role of arecanut (AN) as etiology for oral cancer emerged from Taiwan, where 10% of the population is pure AN chewer and 80% of the preparations do not contain tobacco; on the other side, most of the quid preparation in India contains tobacco.[4]
Tobacco has become a social nuisance now; so, most of the people have switched over to other nontobacco-containing products such as pan-masala that contain AN and lime with other condiments. The other side of the coin is that most of the people including medical professionals are unaware about the side effects of AN: Carcinogenicity and addiction. There are a few in vitro and in vivo studies as well as review articles in the literature stating the role of AN as a carcinogen, but exact carcinogenic pathway has not been clarified yet.[1] This paper intends to present the role of AN as carcinogen, suggest a carcinogenic pathway, and reviews the literature.
AN industry counts almost 300 crore every year; there are 200 billion users; it is openly sold and advertised all over public places without warning.[5] State of California-Environmental protection agency, Office of environmental health hazard assessment-Safe drinking water and toxic enforcement act of 1986 has considered AN as carcinogenic agent in February, 2006.[6] The incidence of oral submucous fibrosis (OSF) from betel nut rages form 0.9 to 4.7% in China, whereas in the India, that is almost up to 0.4 to 10%;[7] and malignant transformation rate of 7.6% in an Indian cohort over a period of 17 years; while in Pakistan, the rate is quite more.[8,9]
In 1969, the International Agency for Research on Cancer (IARC) initiated a program on the evaluation of the carcinogenic risk of chemicals to human beings, involving the production of critically evaluated monographs on individual chemicals. With Supplement 6 (IARC, 1987a), the title of the series was modified from IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans to IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. The criteria established in 1971 to evaluate carcinogenic risk to human beings were adopted by the working groups whose deliberations resulted in the first 16 volumes of the IARC Monographs series. Those criteria were subsequently updated by further ad hoc working groups.[10,11]
In 2003, IARC has considered AN as group 1 human carcinogen. The 2004 monograph includes evaluation of working groups, working procedures, exposure data, etc. The monographs include composition of different substances, industrial packages, geographic region-wise consumption, regulation and legislation, studies of cancer in human and experimental models, physiologic and toxic effects.[10,11]
WHAT IS ARECANUT?
AN (areca catechu—an endosperm (nut/fruit) from tropical tree Areca catechu Linnaeus) is the fourth commonly used psychoactive substance chewed as an aid to digestion and as stimulant, either used alone or added with different tobacco or nontobacco substances to make different combinations. AN is a part of betel quid commonly consumed in Asian countries.[12] “betel nut” is a wrong terminology commonly used for AN; betel tree do not contain fruits but contain only leaves—betel leaves.
AN is known to produce mutagenic and genotoxic effects on tissues of body which may lead to various neoplastic and preneoplastic lesions.[7,11] Commission on cancer (COC) has first considered carcinogenesis of AN in 1993-1994.[13] The target cells of AN are oral fibroblast/myofibroblasts and keratinocytes.[14]
Different types of AN-containing commercially available preparations are available; most of the quids available in India contain tobacco [Table 1]. AN may be used as[11,12] unripe/ripe, whole/sliced, raw/roasted/sun dried, boiled/soaked in water, or fermented (under mud). Depending upon the type of curing, there are many types of AN. Marked reductions in the chemical constituents (carcinogens) were observed when the AN was subjected to soaking and boiling.[13] Boiled nut is due to change in arecanut extract (ANE) composition.[15] In contrast, van Wyk stated that boiled nut contained highest amount of ANE[16] (contents of Arecanut-[Table 2]).
Table 1
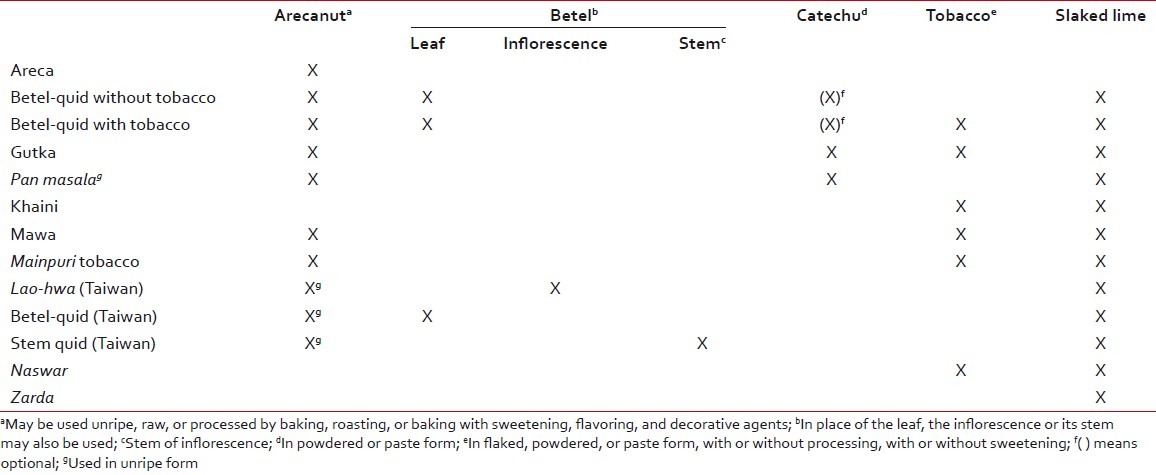
Table 2
Contents of arecanut[11,12]
EPIDEMIOLOGIC STUDIES
AN is consumed widespread in Asia. In countries like India, Pakistan, Bangladesh, Sri Lanka, tobacco is often added, and consumption is higher in women. However, in countries like Taiwan (China) (10% of the population is AN users), Hainan (southern China), and Papua New Guinea (80% of the population is AN users), tobacco is never added. Studies done by Dayal 1978 (1.5% in Ahmedabad mill workers), Gupta 1996 (0.5% in Mumbai), and Daftary 1980 (0.7% in Ernaculam) have reported very less percentage of pure AN chewers. However, on contrast, studies by Chakraborty 1990 (11.4% West Bengal) and Shah 2002 (28.9% Pakistan primary school children) have shown higher rate of consumption.[11]
On the other hand, Taiwanese studies have shown higher AN consumption rate of almost 50%: Tang 1997 (China Hunan 20.3% AN, 15.1% AN with smoking), Ko 1992 (Taiwan 42.1% AN), Yang et al. 2001 (Taiwan 47.8% chewing only, total 69.5%).[11]
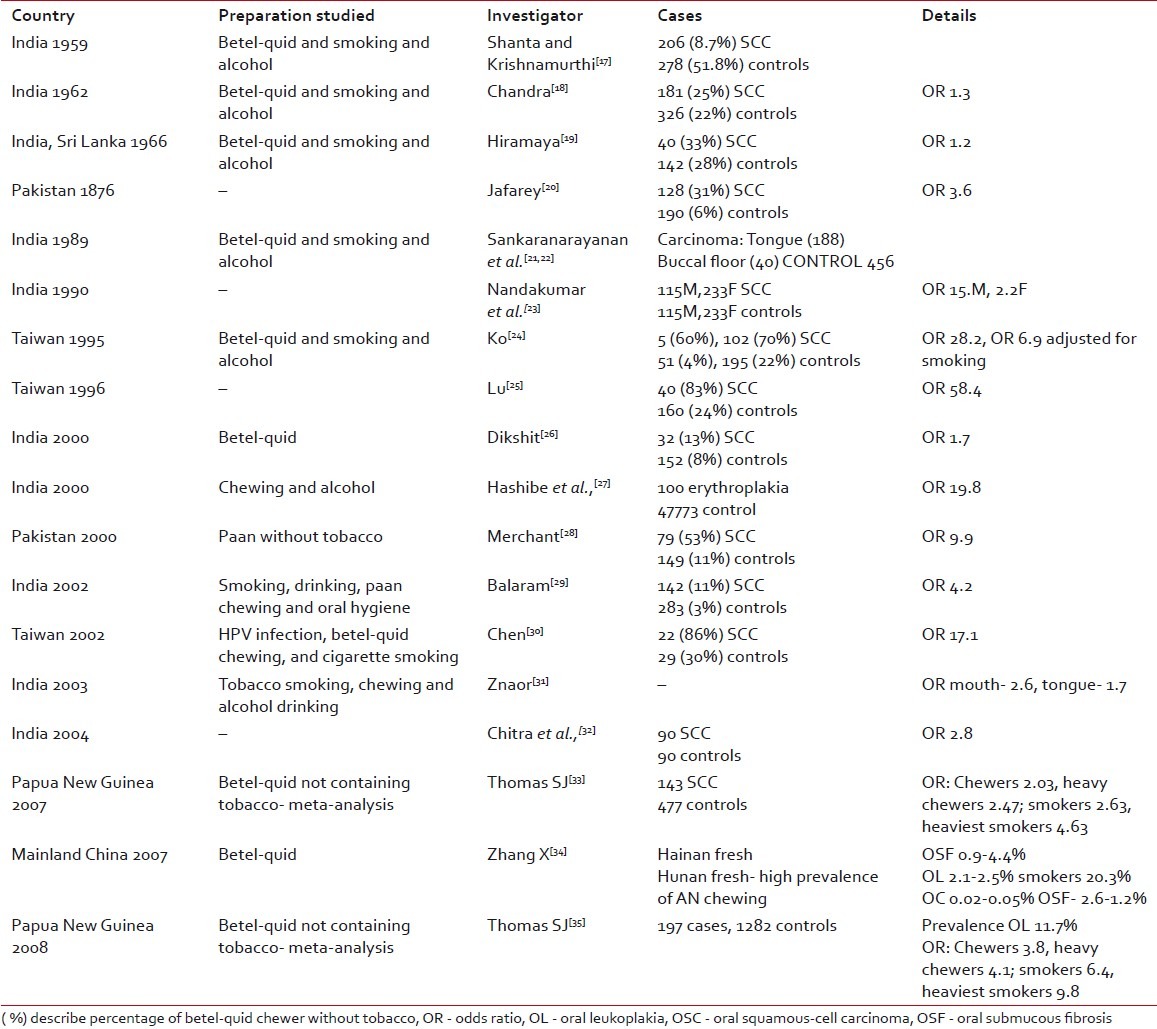
There are so many Indian studies reported in the literature but most of them have studied betel-quid that mostly contain tobacco in Indian scenario. However, some international studies have studied pure AN and there are two meta-analysis. The AN in quid along with tobacco may play synergistic role for carcinogenesis [Table 3].
Table 3
Epidemiologic studies
PSYCHOACTIVE PROPERTIES
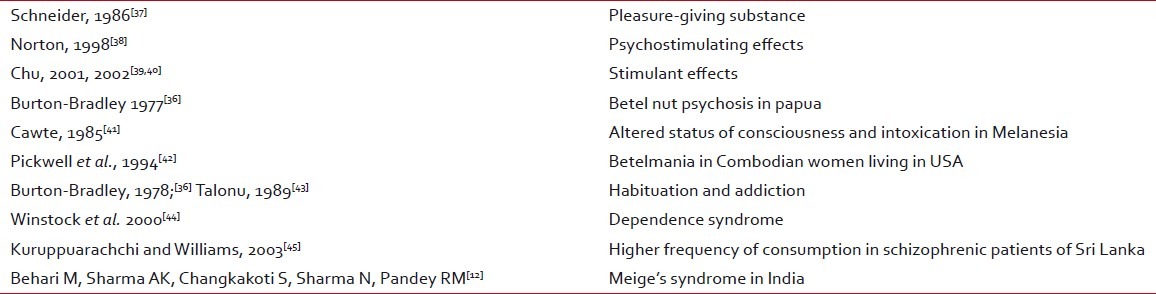
AN is the fourth commonly used psychoactive substance used worldwide, after tobacco, alcohol, and caffeine-containing beverages. AN quid chewing has claimed to produce a sense of well being, euphoria, warm sensations of the body, sweating, salivation, palpitation and heightened alertness, tolerance to hunger, and increased capacity and stamina to work. All these neurological effects suggest that chewing AN quid influences central and autonomic nervous system at various levels.[12] These effects of AN are habit and dose related and is stronger for fresh or occasional chewers than in habitual chewers. Different people have reported different studies regarding this property.[11]
AN psychosis was originally described about 25 years ago in Papua New Guineans by Burton-Bradley (1977). He described how traditional healers challenged victims with so-called betel nut to induce insanity as a part of their diagnostic strategy[11,36] [Table 4].
Table 4
Psychoactive properties
PATHOGENESIS OF CARCINOMA
Emerging evidence indicates that sustained stress exposure induces epigenetic reprogramming of some mammalian cells, thereby increasing mutation rate to accelerate adaptation to stressful environments.[47] ANE has been shown to be mutagenic and genotoxic in a variety of short-term assay systems.
Oral carcinogenesis is a complex, multi-step process that includes initiation, promotion, and progression and is thought to be resulting from the progressive accumulation of genetic lesions after long-term betel-quid (BQ) exposure.[46] Interaction between presumed carcinogens and cellular macromolecules such as DNA, proteins, and lipids is the most important and decisive event of the chemical carcinogenesis.[48,49] Toxicity studies relating to AN-containing polyphenols and tannins are not conclusive, with both carcinogenic and anticarcinogenic effects being reported.[46] Thus, the target organs for tumorigenesis by AN extract and AN polyphenols may be different.[46]
CARCINOGENS IN ARECANUT
The contents that are proven as carcinogens are tannins, some of the polyphenols: Safrole, hydroxychavicol, and catechins, and most of the alkaloids. Some constituents of betel leaf are known to have antimutagenic effects; hydroxychavicol, eugenol.
When the alkaloids are compared on a weight basis with the extract, no single agent has detectable effects on the cells at concentrations of the extract that cause decrease colony survival and DNA single-strand breaks. Therefore, additive or synergistic effects could be considered among the alkaloid.[51]
Alkaloids
Lime is commonly consumed compound along with AN. In the presence of lime (calcium hydroxide), arecoline and guvacoline are hydrolyzed to arecaidine and guvacine. Bacterial enzyme nitrite reductase from denitrifying (Pseudomonas) and non-denitrifying (E. coli, Proteus) bacteria aids in catalysis of nitrosation of secondary amines;[52] and poor oral hygiene also play a role. Thiocyanate in the oral cavity, catechu, and lime also act as a catalyst at pH 9.5. Enhanced by Fe2+, Fe3+, Cu2+, and inhibited by Mn2+.[9] The formation occurs through autoxidation, redox cycling via quinone/semiquinone radical, and iron-catalyzed Haber-Weiss and Fenton reactions.[9]
Arecoline is parasympathomimetic while arecaidine lacks that action. Arecaidine is more potent, cytotoxic, and mutagenic and is tumor promoter. In vitro, this action is prevented by antioxidants such as Glutathione, N- Acetyl L-Cysteine. Arecoline is de-esterified in liver while other compounds are excreted in urine. The metabolic interconversion of arecoline and arecoline 1-oxide is possible.[53] ANE increase salivary flow and decrease pH that may render tissue to more cytotoxic effects.[1]
Nitrosation of arecoline leads to four N-Nitroso compounds: N-Nitrosoguvacoline (NGCO), N-Nitroso guvacine, 3 (Methylnitrosamino) propionitrile (MNPN), 3 (Methylnitrosamino) propionaldehyde.[4,11,13]
These nitroso compounds have been detected in the saliva of AN chewers and are thought to be the culprit of carcinogenesis. Among all these compounds, NGCO is the most significant one. In in vitro studies, MNPN has also shown carcinogenicity.[11,13]
Polyphenols
Polyphenols are likely to contribute to the marked toxicity of the extract. Safrole is also a major component extracted from betel-quid preparation in Taiwan. Its metabolites found in the oral cavity are eugenol and dihydroxychavicol. That had been extendedly studied showing DNA adducts formation in vitro by 32P-postlabeling assay, regarded as a genotoxic carcinogen in the rat liver. Eugenol, a major polyphenol of betel-quid, is cytotoxic to human buccal mucosal fibroblasts by decreasing cellular ATP level and lipid peroxidation. A recent report further suggests role of safrole in oral carcinogenesis, by demonstrating safrole forms, safrole-DNA adducts in human oral tissue following betel-quid chewing.[54]
In contrast, according to some studies, hydroxychavicol and eugenol extracted from betel leaf have antimutagenic effects against dimethylbenzanthracene-induced mutagenesis.[55,56]
MODE OF ACTION
Host defense modulation glutathione
Glutathione is tripeptide involved in detoxification of toxic electrophilic xenobiotics, is reducing agent and antioxidant, and is responsible for cell cycle and thermoregulation.[15]
ANE and polyphenols increase glutathione; while arecoline decrease glutathione; and both decrease protein–sulfhydryl (SH) content. Protein–SH is important for cell division and differentiation and many carcinogens inhibit protein–SH as part of carcinogenesis.[57] ANE decreases GST (glutathione S transferase) and acid soluble sulfhydryl (–SH) levels; while, increases cytochrome b5 and P-450 levels in mice.[58] Thus, they impair host defense.
ANE and arecoline increases PgE2, IL-6, TNF-β in CD4 and CD8 cells, thereby causing impaired T cell activation. In keratinoblasts (KB) cells, these causes COX2 expression and inflammation that leads to decreased cell growth and cell cycle arrest and apoptosis [Figure 1].[59]
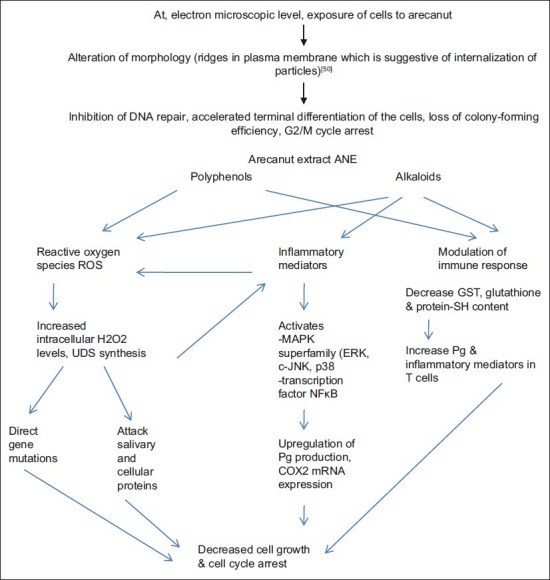
| Fig. 1 Molecular events
Inflammatory mediators prostaglandins
ANE activate mitogen-activated protein kinase superfamily (ERK, c-JNK, p38) and transcription factor NF-κB in oral keratinocytes that are important signaling elements. ANE did not act on EGF receptor signaling system but blockage of NF-κB activation leads to ANE-modulated COX-2 upregulation.[60] But COX-2 mRNA and protein expression upregulation are reversible and can be inhibited by indomethacin and aspirin. Thus, it is not the main pathway.[61] Arecoline induces COX-2 expression in sperm cells in dose-dependent manner and decrease motility.[62]
REACTIVE OXYGEN SPECIES
Various AN constituents may generate reactive oxygen species (ROS) (O2, H2O2, OH) in the presence of lime but catechin fraction is the most active producer. Fe2+ had additive effect, while Mg2+ has marked inhibitory effect.[63] ROS are responsible for oxidative DNA base tissue damage. ROS can be detected by presence of o- and m-tyrosine in saliva of chewer.[64] ANE induces micronuclei and cytokinesis failure in ovary cells in vitro. These changes are associated with increased intracellular H2O2 levels and actin filament disorganization.[65]
In order to provide a defense mechanism against the attack of ROS, cells may exert nonenzymatic and enzymatic systems incorporating agents such as Gluthione S transferase (GSH), catalase, superoxide dismutase (SOD), and glutathione peroxidase, in order to prevent or minimize the toxic damage potentially elicited by ROS.[66,67] ROS acts by (1) Directly gene mutations, (2) Attack salivary proteins and oral mucosa—structural changes—penetration of various objects, (3) Inflammatory cell infiltration—more ROS—mutation of adjacent cells.[46]
ANE-induced unscheduled DNA synthesis (UDS) in gingival keratinocytes may be inhibited by vitamin C, glutathione, desferoxamine (iron chelator and free radical scavenger); while, banthocuproine (copper chelator), 1,10-phenanthroline (lipid permeable iron chelator), and specific reactive oxygen species scavengers such as dimethyl-sulfoxide, mannitol, dimethylthiourea, pyruvate, catalase, and SOD lacked these preventive effects. Higher concentrations of H2O2 inhibited the basal levels of UDS. Thus, it can be stated that these effects are associated with free radical reaction.[68]
However, the extracellular addition of GSH and cysteine has been shown to prevent the arecoline cytotoxicity to cultured OMF in vitro, although SOD and catalase lacked similar preventive effects. This indicates that the cytotoxicity of arecoline to cultured OMF is not mediated by the extracellular production of superoxide radicals and H2O2.[69]
Cell damage
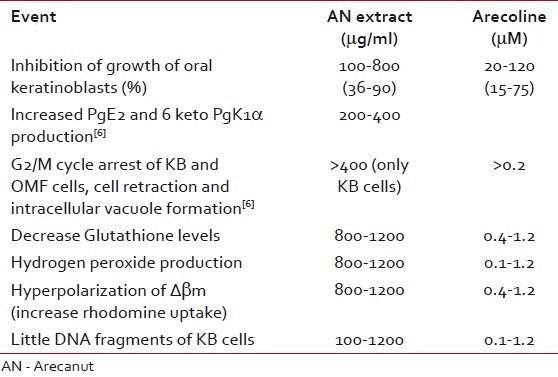
Salivary AN-specific carcinogen 3NPA is known to form DNA protein cross-links and DNA single-strand breaks.[51] Different concentrations of extracts of AN induced dose-dependent UDS in Hep 2 cells. Aqueous and acetic acid extract induce relatively more UDS.[70] Arecoline lowers poly ADP ribosylation in most cellular proteins in Swiss mice. These changes may be the earlier events for initiation of carcinogenesis.[71] Arecoline induced cyclin β1, wee1, phosphorylated CDC 2 protein, and declined p21 expression in KB epithelial cells in gingival Gingival keratinocytes causes the reverse action and ultimately leads to arrest of late S, G2/M cycle. Thus, differential regulation of S and/or G2/M cell cycle-related proteins in the GK and KB cells play a crucial role in different stages of AN-mediated carcinogenesis.[72] ANE is known to cause upregulation of Asb6, a coupling protein to the adapter protein with Pleckstrin homology and Src homology 2 adapter protein, which is involved in insulin signaling for glucose transportation which can be used as prognostic marker.[73] Amount of substance needed for an event is shown in the Table 5.
Table 5
Amount of substance needed for an event
GENES/BIOMARKERS
DNA repair machineries play a pivotal role in maintaining genome integrity. Deregulation of DNA repair can result in genomic instability, which is a hallmark of cancer cells.[74] p53 plays important role in cellular response to stress and is tumor suppressor gene, is the most frequent target (90% involve missense mutation in one allele) for genetic alterations in cancer, and involves in more than 50% of cancers. In Taiwanese, oral cancers infrequent p53 mutations have been reported and 80% of the etiology involves betel-quid which do not contain tobacco in Taiwanese formulations.[75] Some reports from India have also shown infrequent p53 mutation.[76] There is an alternative mechanism of p53 inactivation besides mutations. The mechanism may be either inactivation by abrogating specific DNA binding resulting in p53 sequestering or other genes related to oral cancer (p16/pRb pathway, p21ras, cyclin D1, CD44v7-8, c-myc, N-myc, and Ki-ras).[76]
The role of tissue growth factor (TGF)-β in epithelial malignancy is complex, but it is becoming clear that in the early stages of carcinogenesis, the protein acts as a potent tumor suppressor, while later, TGF-β can function to advance tumor progression.[77] The observed methylation of the p16/MTS1 promoter regions for 54% of tongue squamous-cell carcinoma specimens obtained from BQ-chewers has recently been reported.[78]
ANE induces c-jun proto-oncogene mRNA levels and the effect is independent of glutathione. This may be the mechanism of carcinogenesis.[79,47] Patients with that have poor prognosis. Liu et al. demonstrated presence of safrole DNA adducts in peripheral blood lymphocytes. That can be traced to polymorphism of the CYP2E1 gene, alone and in combination with the GST M1 and GST T1-deletion polymorphisms. Thus, CYP2E1 plays important role for adduct formation.[80]
ANE has shown mutagenicity to S. typhi strains in in vitro studies.[9] It has also induced chromosomal aberrations, sister chromatid exchange, and micronucleated cells and decrease in sperm motility and tumor production in other organs in other in vitro studies.[9]
Arecoline can induce hyperphosphorylation of γ-H2AX which is a marker to examine DNA damage. Upon DNA damage, various molecular events result and ataxia telangiectasia mutated (ATM) kinase plays an important role. Arecoline induces γ-H2AX phosphorylation, triggers ATM-dependant signal pathway and G2/M cycle arrest, suppresses DNA repair, and inhibits expression and transactivation function of p53.[74]
SUBMUCUS FIBROSIS
Pathogenesis is centered with extracellular matrix. Different AN constituents are involved with the collagen production and degradation pathways and they increase collagen production and inhibit collagen degradation thereby causing fibrosis.[81] The reports have shown association between copper content and amount of fibrosis found in other fibrotic disorders such as Wilson's disease, biliary and Indian childhood cirrhosis; upregulation of lysyl oxidase is also seen: An enzyme associated with collagen synthesis and cross-linkage.[82] Upregulation of COX-2 and increased levels of proinflammatory cytokines and reduced levels of anti-fibrotic IFN-γ are also found. Genetic polymorphism is also found to be associated. Increased production of tissue inhibitors of matrix metalloproteinases protein is found in OSF.[83] Autoimmunity is also shown to be involved with that.
CONCLUSION
The pathogenesis of AN carcinogenesis is a complex multistep process involving various pathways and constituents. Carcinogenesis of tobacco is well known and reported in the literature, but no single study is found that has completely supported definite carcinogenesis pathway. Different in vivo and in vitro studies have shown different pathway of carcinogenesis, but when substance inhibiting that particular pathway was used that has not completely inhibited the cellular changes caused by AN substitute. On the other hand, when effects caused by a single AN agent were blocked, even then carcinogenesis was found. So, neither single agent is responsible nor single pathway can produce carcinogenesis and oral submucus fibrosis (OSMF) is related with the carcinogenesis and definite genetic mutations are found to be present.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Molecular events


 PDF
PDF  Views
Views  Share
Share

