Approach to Acute Respiratory Illness in Children with Hematological Malignancy: A Prospective Study Evaluating Utility of CT Scan
CC BY 4.0 · Indian J Med Paediatr Oncol 2022; 43(06): 480-490
DOI: DOI: 10.1055/s-0042-1758539
Abstract
Introduction Various pulmonary complications can occur in children with hematological malignancies including both infection and malignant disease infiltration of pulmonary parenchyma.
Objectives To assess the role of CT scan in determining the etiology of acute pulmonary complications in children with hematological malignancies.
Materials and Methods All children < 17 years with newly diagnosed hematological malignancy with respiratory symptoms (Group A) along with children who developed fever with persistent respiratory symptoms as well as worsening chest radiographs during treatment (Group B) and underwent CECT thorax, from February 2019 to July 2020 were enrolled. The final diagnosis was made on the basis of clinical history, laboratory as well as radiological investigations and treatment response.
Results Thirty-seven children with mean age of 7.5 ± 3.5 years and male to female ratio of 1.3:1 who underwent CECT thorax were included in our study. For newly diagnosed cases, i.e., Group A (n = 8), the most common cause of respiratory symptoms as identified on CECT thorax was pulmonary tumoral infiltration (n = 5) followed by tuberculosis (n = 3). However, in Group B (n = 29) the cause of persistent respiratory symptoms was identified as infection (n = 17) followed by leukemic infiltration (n = 12). Thus, chest CT could accurately identify pulmonary tuberculosis, fungal pneumonia, bacterial infection, and pulmonary tumoral infiltrates.
Conclusion CT scan can be used as an adjunctive tool for prompt diagnosis and management of pulmonary complications in children with persistent respiratory symptoms as they are often non-specific.
Data, Materials and/or Code Availability
Data are available with the corresponding author and can be shared on reasonable request.
Ethics Approval
The study was approved by the institutional ethics committee of Institute of medical Sciences, Banaras Hindu University.
Publication History
Article published online:
29 November 2022
© 2022. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction Various pulmonary complications can occur in children with hematological malignancies including both infection and malignant disease infiltration of pulmonary parenchyma.
Objectives To assess the role of CT scan in determining the etiology of acute pulmonary complications in children with hematological malignancies.
Materials and Methods All children < 17 years with newly diagnosed hematological malignancy with respiratory symptoms (Group A) along with children who developed fever with persistent respiratory symptoms as well as worsening chest radiographs during treatment (Group B) and underwent CECT thorax, from February 2019 to July 2020 were enrolled. The final diagnosis was made on the basis of clinical history, laboratory as well as radiological investigations and treatment response.
Results Thirty-seven children with mean age of 7.5 ± 3.5 years and male to female ratio of 1.3:1 who underwent CECT thorax were included in our study. For newly diagnosed cases, i.e., Group A (n = 8), the most common cause of respiratory symptoms as identified on CECT thorax was pulmonary tumoral infiltration (n = 5) followed by tuberculosis (n = 3). However, in Group B (n = 29) the cause of persistent respiratory symptoms was identified as infection (n = 17) followed by leukemic infiltration (n = 12). Thus, chest CT could accurately identify pulmonary tuberculosis, fungal pneumonia, bacterial infection, and pulmonary tumoral infiltrates.
Conclusion CT scan can be used as an adjunctive tool for prompt diagnosis and management of pulmonary complications in children with persistent respiratory symptoms as they are often non-specific.
Introduction
Acute respiratory illness (ARI) in immunocompromised children remains a significant diagnostic challenge for both clinicians and radiologists. Pulmonary infection complicates ∼75% of immunocompromised patients during their treatment.[1] Given the innumerable pathogens that can cause infectious complications in immunocompromised children, identifying a specific pathogen can be elusive.
In children developing complications secondary to chemotherapy-induced immune suppression, when combined with certain specific morphological findings and the type and duration of immune suppression, radiological investigations can aid with the decision-making process. Chest radiography is the initial imaging modality of choice for the diagnostic assessment of patients presenting with ARI; however, there is low sensitivity and low specificity regarding the type of specific pathogens.[2] Chest CT has been demonstrated to confer a higher degree of sensitivity and specificity for identifying the underlying cause of pulmonary involvement.
Contrast-enhanced CT chest should be done to evaluate the lungs, mediastinum, and pleural/chest wall abnormalities and identify mediastinal and hilar lymph nodes, as opposed to axial HRCT (high-resolution CT), which is done for pulmonary pathologies only. Volumetric multidetector CT acquisition in most of the modern day scanners can generate reconstructed HRCT images, which obviate the use of traditional axial HRCT acquisition in pediatric patients. Apart from pulmonary infections, non-infectious causes such as malignant pulmonary infiltration, radiation-induced lung injury, pulmonary thromboembolic phenomenon, should also be considered while evaluating acute respiratory illness in this group.
The aim of this study was to assess the role of contrast-enhanced CT scan in determining the etiology of pulmonary complications in children with hematological malignancies presenting with ARI.
Materials and Methods
This was a prospective observational study performed at the Division of Pediatric Hematology Oncology, Department of Pediatrics, Institute of Medical Sciences, Banaras Hindu University from February 2019 to July 2020 (ethical clearance no. Dean/2018/EC/919). Thirty-seven children were included in this study who met our inclusion criteria.
Inclusion Criteria
All children < 17 years with hematological malignancy who were
 Newly diagnosed with respiratory symptoms (group A)
Newly diagnosed with respiratory symptoms (group A) On chemotherapy and developed fever with persistent respiratory symptoms, i.e., respiratory symptoms present even after 7 to 10 days from start of treatment along with/without worsening chest radiographs (group B)
On chemotherapy and developed fever with persistent respiratory symptoms, i.e., respiratory symptoms present even after 7 to 10 days from start of treatment along with/without worsening chest radiographs (group B)
Exclusion Criteria
 Unwillingness to participate in the study
Unwillingness to participate in the study Hemodynamically unstable child
Hemodynamically unstable child
Children were investigated and empirical intravenous antibiotics, i.e., piperacillin-tazobactam and amikacin were started as per unit protocol. Investigations such as complete blood count, conventional blood culture, chest X-ray were sent for all children that had either of the two, i.e., fever, cough, or increased respiratory rate for age. Intravenous/oral fluconazole (antifungal) was added after 48 to 72 hours3 if the child had clinical deterioration despite receiving empirical intravenous antibiotics. As pulmonary infection in immunocompromised children is most likely due to bacterial pathogens,[2] antibiotics was started and resolution of symptoms were expected to occur by 7 to 10 days (arbitrary) with antibiotics, followed by antifungals (as indicated) as per blood culture sensitivity pattern in the pediatric oncology ward. Patients responding to antibiotics in both the groups and having a normal follow-up chest X-ray at 7 to 10 days did not undergo chest CT. In both the groups, if after 7 to 10 days, there was no clinical improvement to empirical antibiotics, a repeat conventional blood culture, chest X-ray, and chest CT was done. The indications of chest CT in our study were persistent respiratory symptoms, irrespective of abnormality on chest X-ray at 7 to 10 days of antibiotics.
Chest CT findings were further corroborated by blood culture, gastric aspirate for Gene Xpert and galactomannan assay (based on availability) in infective cases. As gastric aspirate in children has been found to be equally effective and beneficial in Mycobacterium tuberculosis (MTB) detection with non-requirement for specific facilities as compared with bronchoalveolar lavage, it was used in this study for MTB detection.[3] CT-guided tissue biopsy from mediastinal mass was performed in most cases where malignant infiltration was suspected. Children whose parents did not consent for tissue biopsy, it was not done. Pulmonary infiltration was suspected if the clinical condition of the child was consistent with disease infiltration and child had sterile cultures. However, lung biopsy was not performed.
Data were collected with respect to age, gender, duration of symptoms, radiological findings on chest X-ray and chest CT, along with response to treatment. In infective cases, results of blood culture, gastric aspirate for Gene Xpert and galactomannan assay (based on availability) along with results of tissue biopsy in cases where malignant infiltration was suspected were also collected.
CECT Protocol
CT chest was performed using a 128 slice light speed VCT (GE Medical System). Non-ionic contrast agent Iohexol (Omnipaque) of concentration 300 mgI/mL was administered using hand injector (with an optional use of power injector and bolus chase) in a calculated dosage(1–1.5 mL/kg) depending upon the child's weight. In patients with previous history of contrast allergy or high-risk of contrast allergy (previous anaphylactic reactions to food or medication), after injecting the first 1 mL of contrast agent, child was observed for a few seconds for any allergic reactions and again after half an hour to see any delayed reaction. Scan delay of 25 to 30 seconds was kept following the administration of contrast for optimal enhancement of soft tissue. Images were then reconstructed using different reconstruction algorithm and evaluated in advantage workstation 4.4 and 4.7.
Image Evaluation and Interpretation
All CT scans were evaluated by a radiologist with 7 years of experience. Following definitions were used for characterizing lesions seen on CT scan.[4]
Consolidation is defined as homogenous increase in lung parenchymal attenuation with obscuration of airways and vessels.
Ground glass opacities (GGO) are defined when bronchovascular margins are preserved behind hazy area of homogenously increased attenuation.
A nodule around the peripheral pulmonary arterial branches or 3 to 5 mm from the pleura, pulmonary vein or interlobular septa is defined as centrilobular nodule. Appearance of multiple areas of centrilobular nodules with a linear branching pattern was defined as “tree-in-bud” nodules. Nodules were termed as random if they lacked an architectural predominance.
Abnormal widening of the interlobular septa is defined as an interlobular septal thickening.
Each parenchymal lesion localized to a particular lobe was evaluated in terms of presence and distribution of abnormality identified on CECT thorax.
Halo sign was defined as ground-glass opacity surrounding a nodule or, a mass or a rounded area of consolidation.
Approach to Diagnosis
Following signs on CT were used to suggest the specific etiology of pulmonary findings.
Bacterial pneumonia: Patients with segmental or lobar consolidation with/without pleural effusion
Tuberculosis: Centrilobular nodules with tree in bud pattern, necrotizing mediastinal and hilar lymph nodes, empyema, cavitatory consolidations, military pattern, and cavitatory lesions.
Fungal infection: Patchy consolidations and halo sign, cavitatory nodules, consolidation with crescent of air.
Viral pneumonia: bilateral multifocal GGO with patchy consolidations with perihilar distribution along bronchovascular bundles indicative of bronchopneumonia.
Lymphoma/leukemic infiltrate: mediastinal mass with lung consolidation or randomly distributed nodule with bronchovascular bundle thickening or nodular pleural thickening
All children were followed and a repeat CECT was performed after the completion of treatment in children with tuberculosis and malignant pulmonary infiltration. The final etiological diagnosis was made on the basis of clinical history, laboratory investigations and treatment response was considered as an outcome measure in this study.
Statistical Analysis: SPSS software (Version 22.0. IBM Corp. Armonk, NY, USA) for windows was used for data entry and analysis. All numerical variables are expressed as median with range. For comparison of categorical data chi square test was used. For categorical variables with cell values < 5, Fisher's exact test was used. A p-value < 0.05 was taken as significant.
Ethics: The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1964, as revised in 2013. Ethical clearance from Institute Ethical Committee of Institute of Medical Sciences, Banaras Hindu University, was obtained (Dean/2018/EC/919). Written informed consent was obtained from parents of all children included in our study.
Results
During the study period, a total of 103 children developed ARI and were divided in two separate groups ([Fig. 1]). The first group (Group A) comprised of newly diagnosed leukemia/lymphoma (59.2% [n = 61]). Among them, 29.5% (n = 18) had respiratory symptoms at the start of treatment, of which 50% (n = 9) improved with empirical antibiotics, 5% (n = 1) abandoned treatment, and 44.4% (n = 8) underwent CECT thorax. The second group (Group B) consisted of children who developed respiratory symptoms while on different phases of chemotherapy 40.8% (n = 42). Among them, 14.3% (n = 6) improved with empirical antibiotics, 16.7% (n = 7) abandoned treatment, and 69% (n = 29) underwent CECT thorax. Our study included a total of 37 children (mean age 7.5 ± 3.5 years; male to female ratio of 1.3:1) who did not show improvement after primary treatment and had persistent respiratory symptoms. Of these 37 children, underlying hematological malignancy included acute lymphoblastic leukemia in 43.2% (n = 16), non-Hodgkin lymphoma in 24.3% (n = 9), acute myeloid leukemia in 21.6% (n = 8), and Hodgkin's lymphoma in 10.8% (n = 4) ([Table 1]).
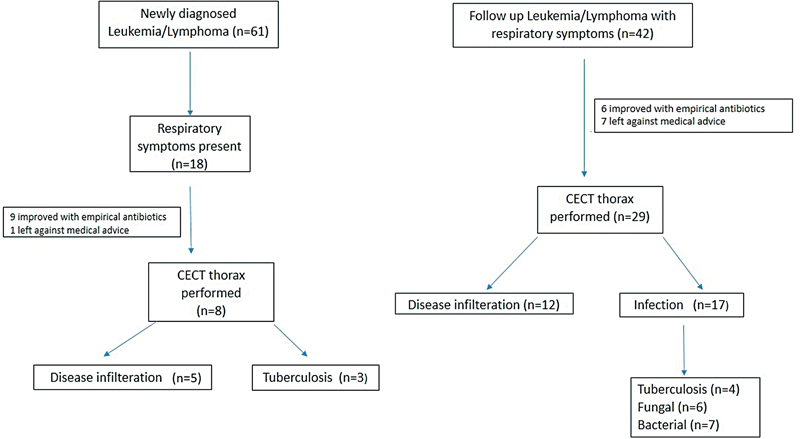
| Figure 1:Flow diagram depicting patient enrolment.
|
Characteristics |
|
|---|---|
|
Age |
7.5 ± 3.5 years |
|
M:F ratio |
1.3:1 |
|
Underlying pathology: n (%) |
|
|
ALL |
16 (43.2%) |
|
AML |
8 (21.6%) |
|
NHL |
9 (24.3%) |
|
HL |
4 (10.8%) |
|
Presenting symptoms: n (%) |
|
|
Fever |
31 (83.7%) |
|
Cough |
17 (45.9%) |
|
Rapid breathing |
12 (32.4%) |
|
Chest pain |
1 (0.02%) |
|
Etiological cause: n (%) |
|
|
Bacterial |
7 (18.9%) |
|
Fungal |
6 (16.2%) |
|
Tuberculosis |
7 (18.9%) |
|
Leukemic infiltration/disease |
17 (46%) |
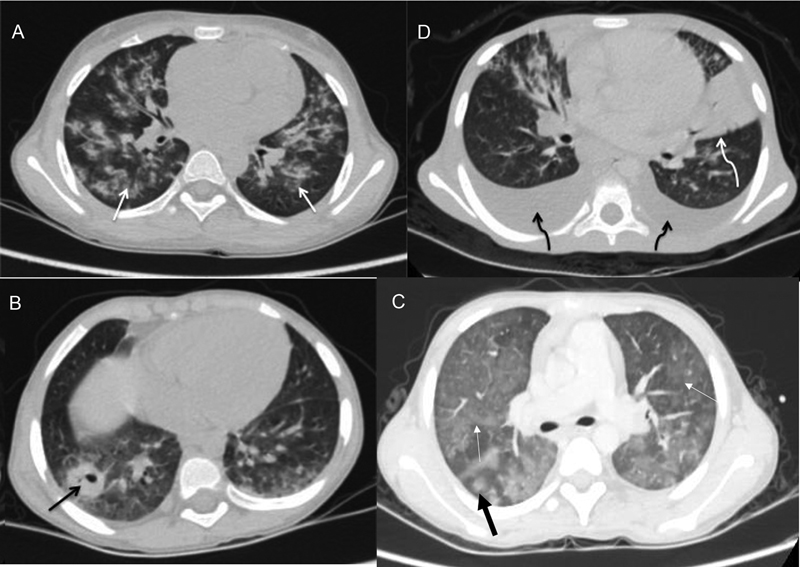
| Figure 2:Axial CECT images of four different patients showing (A) bilateral pulmonary infiltrates in form of centrilobular nodules (white arrows) with adjacent ground glass opacities representing tubercular involvement (B) bilateral pleural effusion (black curved arrow) and lobar consolidation in left lingular segments (white curved arrow) in bacterial infection and (C) cavitatory lesion in the right lower lobe (black arrow) suggestive of fungal infection. (D) Bilateral ground glass opacities (white arrows) with halo sign (thick black arrow) in fungal infection.
|
S.no |
Age (y) |
Diagnosis |
Clinical presentation |
Investigations |
CECT findings |
Treatment |
Follow-up |
|
|---|---|---|---|---|---|---|---|---|
|
Chest X-ray (day 7) |
||||||||
|
1 |
7/M |
ALL with pulmonary tuberculosis |
Fever cough Rapid breathing for 20 days |
Patchy opacity in right upper lobe, B/L CP angle bluting |
Pleural R/M-high ADA lymphocytosis (91%) |
Patchy consolidation along with ground glass opacities, centrilobular nodules and bilateral pleural effusion was observed |
Empirical antibiotics On the basis of CECT and pleural tap R/M suggesting TB, ATT was advised for 6 months |
Clinical improvement observed following ATT |
|
2 |
9/M |
AML with fungal pneumonia |
Cervical lymphadenopathy for 15 days Fever and cough for 5 days |
Normal |
Galactomannan assay- positive |
Focal ground glass opacities with surrounding consolidation (reverse halo sign) and focal patchy opacity were observed |
Empirical antibiotics Amphotercin B administered for 14 days. |
Fever subsided and child clinically improved |
|
3 |
12/M |
ALL with fungal pneumonia /septic pulmonary embolism |
Fever (during induction phase of chemotherapy) |
Focal patchy opacities and left sided loculated effusion |
Blood C/S was positive for MRSA |
Randomly distributed cavitary and non-cavitary nodule with left sided empyema and peripheral patchy consolidation with surrounding GGO were observed |
Empirical antibiotics along with Amphotericin B. |
Clinical response after Vancomycin administration |
|
4 |
3/M |
ALL with pulmonary tuberculosis |
Cough and neck swelling for 15 days fever for 2 days |
Opacity in right middle lobe and infiltrative pattern |
Blood culture-sterile Gene Xpert- Negative |
Multiple bilateral centrilobular nodule with tree in bud appearance, focal GGO and right middle lobe atelectasis was observed |
Empirical antibiotics Anti-tubercular drug was advised for 6 months |
Clinical improvement observed after ATT intake |
|
5* |
4/M |
ALL with pulmonary tuberculosis |
on/off fever, Rapid breathing and cervical lymphadenopathy |
Multiple reticular opacities |
Blood C/S- sterile Gene Xpert- Negative |
Multiple patchy consolidation in bilateral upper lobe with multiple ground glass opacities, centrilobular nodules with tree in bud appearance |
ATT was advised for 6 months |
Clinical improvement observed after ATT intake |
|
6 |
2/F |
ALL with pulmonary tuberculosis |
Fever and cough for 20 days History of significant weight loss |
Inhomogenous opacity in Right upper zone |
Gene X-pert-negative |
Sublobar consolidation in right upper lobe, pleural effusion and mediastinal lymph nodes |
Empirical antibiotics ATT was advised for 6 months |
Clinical improvement observed after ATT intake. |
|
7* |
3/F |
ALL with pulmonary tuberculosis |
On/off fever for 22 days and cough for 5 days |
ight lung infiltrates |
Blood C/S - sterile Gene X-pert- positive. |
Diffuse ground glass opacities and patchy consolidation in bilateral upper and lower lobe, centrilobular nodule showing tree in bud appearance in B/L lower lobes and mediastinal lymph node was observed |
Empirical antibiotics ATT advised |
Child died due to progressive disease |
|
8 |
6/M |
NHL with Bronchopneumonia |
On/off fever for 1 month |
Normal |
Blood culture sterile |
Lobar consolidation with centrilobular ground glass opacities and sub-centrimetric mediastinal lymph nodes |
Empirical antibiotics |
Fever improved following meropenem and vancomycin administration |
|
9 |
1/F |
AML with fungal pneumonia |
On/off fever for 15 days |
Normal |
Blood c/s- sterile Gene X-pert- negative Galactomannan assay- Not done |
Focal diffuse GGO and centrilobular nodule and mild pleural effusion s/o infective etiology |
Empirical antibiotics |
Child improved clinically after amphotericin B administration |
|
10 |
4/M |
NHL with bronchopneumonia |
Fever and cough for 15 days |
Bilateral inhomogenous opacities |
Blood c/s sterile Gene X-pert- negative. |
B/L lobar consolidation with minimal pleural effusion was noted s/o infective cause |
Empirical antibiotics |
Clinical improvement after Teicoplanin and meropenem administration |
|
11 |
12/M |
ALL with fungal pneumonia |
Fever for 12 days |
Inhomogenous opacity in right mid zone |
Blood c/s –candida Galactomannan assay- Not done |
Randomly distributed multiple nodules with surrounding GGO and patchy consolidation in right middle lobe, subcentrimetric mediastinal lymph node and minimal pleural effusion noted |
Empirical antibiotics Amphotericin-B administered following CECT findings and culture report |
Clinical improvement after amphotericin B administration |
|
12 |
4/M |
ALL with Septic pulmonary embolism Vs fungal infection |
Fever for 15 days |
Normal |
Blood C/S sterile Gene X-pert- negative Galactomannan assay positive |
Multiple cavitary lesion with surrounding consolidation in B/L lung field with mediastinal lymph node |
Empirical antibiotics with Fluconazole |
Child improved after day 4 of fluconazole administration. |
|
13 |
13/M |
ALL with fungal pneumonia |
Child fever for 20 days and cough for 15 days. |
Bilateral inhomogenous opacities |
Blood culture –candida Galactomannan assay not done |
Segmental consolidation with surrounding GGO in left upper lobe, and few centrilobular nodule with tree in bud appearance, and minimal pleural effusion |
Empirical antibiotics with amphotericin B was administered for 2 weeks |
Clinical improvement after amphotericin B administration |
|
14* |
7/M |
ALL with fungal pneumonia |
Fever for 10 days and poor oral intake for 1 month |
Normal |
Blood culture sterile Gene X-pert- positive |
Multiple random nodules with surrounding GGO, mild interstitial thickening and mediastinal lymph node |
Empirical antibiotics with fluconazole. ATT advised following CT findings and positive Gene Xpert |
Child improved following ATT administration. |
|
15 |
5/F |
AML with fungal pneumonia |
Cough and rapid breathing for 4 days |
Focal opacity |
Blood C/S sterile Gene X-pert- negative Galactomannan positive |
Patchy consolidation with surrounding GGO, focal and diffuse GGO and randomly distributed nodule was observed |
Empirical antibiotics with fluconazole |
Child responded to voriconazole |
|
16 |
6/M |
AML with bronchopneumonia |
Fever and cough for 12 days |
Consolidation, CP angle blunting |
Blood c/s–positive for coagulase negative staphylococcus |
Lobar consolidation with pleural effusion and mediastinal lymph node was observed |
Empirical antibiotics with fluconazole vancomycin was advised as per blood culture sensitivity |
Clinical improvement after Vancomycin administration |
|
17 |
16/F |
AML with pulmonary tuberculosis. |
Fever, cough for 25 days and rapid breathing for 10 days |
Normal |
Blood culture sterile Gene X-pert negative |
Centrilobular nodule with tree in bud appearance and pleural effusion |
Empirical antibiotics with fluconazole ATT was advised based on CECT findings and no response to antibiotics as well as antifungals |
.Clinically improvement following ATT intake for 6 months |
|
18 |
12/F |
AML with bronchopneumonia |
Fever and rapid breathing for 4 days |
Inhomogenous opacities |
Blood culture – MRSA** |
Segmental consolidation with pleural effusion, few centrilobular nodule and subcentrimetric mediastinal lymph node |
Empirical antibiotics with fluconazole Vancomycin was advised as per blood culture sensitivity |
Clinical improvement after vancomycin administration |
|
19 |
8/F |
ALL with bronchopneumonia |
Fever and rapid breathing for 10 days |
Consolidations |
Blood culture - E. coli. |
B/L segmental consolidation with peri bronchovascular nodule and few subcentrimetric mediastinal lymph node |
Empirical antibiotics Imipenem was advised as per blood culture sensitivity |
Clinical improvement after Imipenem administration |
|
20 |
9/F |
AML |
Fever and cough for 3 days |
Consolidations |
Blood c/s sterile Gene X-pert - negative |
B/L lobar consolidation with pleural effusion and mediastinal lymph node. |
Broad spectrum antibiotics was started. |
Responded to the treatment. |
|
S no. |
Age (y)/ Sex |
Diagnosis |
Clinical presentation |
Investigations |
CECT findings |
|
|---|---|---|---|---|---|---|
|
Chest X-ray |
||||||
|
1 |
8/F |
Relapsed ALL |
On/off fever, neck swelling, cough and rapid breathing |
Hyperleukocytosis |
Normal |
Multiple diffusely spread patchy ground glass opacities (GGO) with mild interstitial thickening in bilateral lungs and moderate pleural effusion and enlarged mediastinal lymph node. |
|
2 |
13/M |
Relapsed ALL |
Fever and difficulty in breathing |
Blood C/S - Sterile Gene X-pert - negative |
Normal |
Subpleural patchy opacities with interstitial thickening, and subcentrimetric random nodules |
|
3 |
6/F |
Relapsed ALL |
On/off fever and lymphadenopathy |
Hyperleukocytosis |
Normal |
Multiple randomly distributed nodule with surrounding GGO, nodular thickening of interlobular fissure and enlarged mediastinal lymph node |
|
4 |
10/M |
Relapsed ALL |
On/off fever and cough |
Blood C/S - sterile |
Widened mediastinum, Inhomogenous opacities |
Multiple mediastinal lymph node with subsegmental patchy consolidation, random nodules and focal GGO |
|
5 |
11/M |
Relapsed ALL |
On/off fever and rapid breathing |
Blood C/S - Sterile |
Mediastinal widening |
Conglomerated mediastinal lymph nodes with adjacent subsegmental consolidation and pericardial effusion, randomly distributed multiple soft tissue density nodule, mild pleural thickening and bony erosion of sixth rib. |
|
6* |
2/F |
NHL |
Fever and lymphadenopathy |
Blood C/S - Sterile |
Right upper lobe opacity. Widened mediastinum |
Near complete thrombosis of SVC, proximal right subclavian and B/L brachiocephalic vein. Randomly distributed nodule with few showing surrounding GGO, right upper lobe segmental consolidation and bilateral pleural effusion with mild pleural thickening. Few subcentrimetric mediastinal lymph nodes. |
|
7 |
11/M |
Relapsed AML |
Fever and cough |
Blood C/S - Sterile Gene X-pert - negative |
Inhomognoeus opacity |
Focal patchy consolidation, multiple diffuse patchy GGO with mild interstitial thickening and subcentrimetric mediastinal lymph nodes. |
|
8* |
13/M |
NHL |
Fever chest pain and rapid breathing |
Lung opacity, CP angle blunting |
Anterior and superior mediastinal mass with right upper lobe consolidation and moderate pleural effusion. |
|
|
9* |
9/M |
HL |
Fever, neck swelling for 7 months |
Blood C/S - Sterile Gene X-pert - Negative. |
Inhomogneous opacity |
Patchy consolidation, bilateral nodular pleural thickening and mediastinal lymph nodes |
|
10 |
5/M |
Refractory NHL |
Fever |
Inhomogneous opacity |
Focal subsegmental consolidation, Soft tissue density random nodule and hilar lymph nodes. |
|
|
11* |
8/M |
HL |
Fever, rapid breathing and lymphadenopathy |
Consolidation with inhomognoeus opacity |
Subsegmental consolidation, centrilobular nodule with surrounding mild GGO with mediastinal lymph node |
|
|
12 |
4/F |
Refractory NHL |
Rapid breathing |
Bronchopneumonia, bilateral CP angle blunting |
Segmental consolidation, bilateral nodular pleural thickening with pleural effusion and mediastinal lymph nodes |
|
|
13 |
6/F |
Refractory HL |
Fever, rapid breathing and lymphadenopathy |
. |
Mediastinal widening |
Large mildly enhancing mediastinal mass with necrosis, nodular thickening of pleura with pleural effusion |
|
14 |
6/F |
Relapsed NHL |
On/off fever, cough and rapid breathing |
Mediastinal widening |
Mediastinal enhancing mass occupying superior and anterior mediastinum, pleural thickening and mediastinal lymph nodes |
|
|
15 |
8/F |
Refractory NHL |
Fever and rapid breathing. |
Hyperleukocytosis |
Bilateral inhomogenous opacities |
Segmental consolidation, B/L diffuse focal GGO with interstitial thickening and calcified enlarged mediastinal lymph nodes |
|
16 |
7/M |
RefractoryNHL |
Fever, cough and rapid breathing |
Blood C/S - Sterile Gene X-pert - negative. |
Mediastinal widening. |
Anterior mediastinal enhancing mass with bilateral minimal pleural and pericardial effusion. Patchy ground glass opacities present |
|
17* |
7/F |
HL |
Neck swelling for 6 months |
Blood C/S - Sterile Gene X-pert - Negative |
Inhomogenous opacities |
Middle lobe segmental consolidation, halo sign and cervical non-necrotic lymph nodes |
References
- Cereser L, Zuiani C, Graziani G. et al. Impact of clinical data on chest radiography sensitivity in detecting pulmonary abnormalities in immunocompromised patients with suspected pneumonia. Radiol Med (Torino) 2010; 115 (02) 205-214
- Lee C, Colletti PM, Chung JH. et al; Expert Panel on Thoracic Imaging. ACR Appropriateness Criteria® acute respiratory illness in immunocompromised Patients. J Am Coll Radiol 2019; 16 (11S): S331-S339
- Baghaei P, Tabarsi P, Farnia P. et al. Utility of gastric lavage for diagnosis of tuberculosis in patients who are unable to expectorate sputum. J Glob Infect Dis 2011; 3 (04) 339-343
- Davis K, Wilson S. Febrile neutropenia in paediatric oncology. Paediatr Child Health (Oxford) 2020; 30 (03) 93-97
- Copley SJ. Application of computed tomography in childhood respiratory infections. Br Med Bull 2002; 61 (01) 263-279
- Jung JI, Lee DG, Kim YJ, Yoon HK, Kim CC, Park SH. Pulmonary tuberculosis after hematopoietic stem cell transplantation: radiologic findings. J Thorac Imaging 2009; 24 (01) 10-16
- Demirkazik FB, Akin A, Uzun O, Akpinar MG, Ariyürek MO. CT findings in immunocompromised patients with pulmonary infections. Diagn Interv Radiol 2008; 14 (02) 75-82
- Nam BD, Kim TJ, Lee KS, Kim TS, Han J, Chung MJ. Pulmonary mucormycosis: serial morphologic changes on computed tomography correlate with clinical and pathologic findings. Eur Radiol 2018; 28 (02) 788-795
- Franquet T, Giménez A, Hidalgo A. Imaging of opportunistic fungal infections in immunocompromised patient. Eur J Radiol 2004; 51 (02) 130-138
- Brook O, Guralnik L, Hardak E. et al. Radiological findings of early invasive pulmonary aspergillosis in immune-compromised patients. Hematol Oncol 2009; 27 (02) 102-106
- Austin JH, Müller NL, Friedman PJ. et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996; 200 (02) 327-331
- Tanaka N, Matsumoto T, Miura G. et al. CT findings of leukemic pulmonary infiltration with pathologic correlation. Eur Radiol 2002; 12 (01) 166-174
- Heyneman LE, Johkoh T, Ward S, Honda O, Yoshida S, Müller NL. Pulmonary leukemic infiltrates: high-resolution CT findings in 10 patients. AJR Am J Roentgenol 2000; 174 (02) 517-521
- Reynolds JH, Banerjee AK. Imaging pneumonia in immunocompetent and immunocompromised individuals. Curr Opin Pulm Med 2012; 18 (03) 194-201
- Kisembo HN, Boon SD, Davis JL. et al. Chest radiographic findings of pulmonary tuberculosis in severely immunocompromised patients with the human immunodeficiency virus. Br J Radiol 2012; 85 (1014): e130-e139
- Heussel CP, Kauczor HU, Heussel G, Fischer B, Mildenberger P, Thelen M. Early detection of pneumonia in febrile neutropenic patients: use of thin-section CT. AJR Am J Roentgenol 1997; 169 (05) 1347-1353
Address for correspondence
Ishan Kumar, MBBS, MD, DNBDepartment of Radiodiagnosis, Institute of Medical Sciences, Banaras Hindu UniversityVaranasi 221005, Uttar PradeshIndiaEmail: ishan.imsrd@bhu.ac.inPublication History
Article published online:
29 November 2022© 2022. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Flow diagram depicting patient enrolment.

| Figure 2:Axial CECT images of four different patients showing (A) bilateral pulmonary infiltrates in form of centrilobular nodules (white arrows) with adjacent ground glass opacities representing tubercular involvement (B) bilateral pleural effusion (black curved arrow) and lobar consolidation in left lingular segments (white curved arrow) in bacterial infection and (C) cavitatory lesion in the right lower lobe (black arrow) suggestive of fungal infection. (D) Bilateral ground glass opacities (white arrows) with halo sign (thick black arrow) in fungal infection.
References
- Cereser L, Zuiani C, Graziani G. et al. Impact of clinical data on chest radiography sensitivity in detecting pulmonary abnormalities in immunocompromised patients with suspected pneumonia. Radiol Med (Torino) 2010; 115 (02) 205-214
- Lee C, Colletti PM, Chung JH. et al; Expert Panel on Thoracic Imaging. ACR Appropriateness Criteria® acute respiratory illness in immunocompromised Patients. J Am Coll Radiol 2019; 16 (11S): S331-S339
- Baghaei P, Tabarsi P, Farnia P. et al. Utility of gastric lavage for diagnosis of tuberculosis in patients who are unable to expectorate sputum. J Glob Infect Dis 2011; 3 (04) 339-343
- Davis K, Wilson S. Febrile neutropenia in paediatric oncology. Paediatr Child Health (Oxford) 2020; 30 (03) 93-97
- Copley SJ. Application of computed tomography in childhood respiratory infections. Br Med Bull 2002; 61 (01) 263-279
- Jung JI, Lee DG, Kim YJ, Yoon HK, Kim CC, Park SH. Pulmonary tuberculosis after hematopoietic stem cell transplantation: radiologic findings. J Thorac Imaging 2009; 24 (01) 10-16
- Demirkazik FB, Akin A, Uzun O, Akpinar MG, Ariyürek MO. CT findings in immunocompromised patients with pulmonary infections. Diagn Interv Radiol 2008; 14 (02) 75-82
- Nam BD, Kim TJ, Lee KS, Kim TS, Han J, Chung MJ. Pulmonary mucormycosis: serial morphologic changes on computed tomography correlate with clinical and pathologic findings. Eur Radiol 2018; 28 (02) 788-795
- Franquet T, Giménez A, Hidalgo A. Imaging of opportunistic fungal infections in immunocompromised patient. Eur J Radiol 2004; 51 (02) 130-138
- Brook O, Guralnik L, Hardak E. et al. Radiological findings of early invasive pulmonary aspergillosis in immune-compromised patients. Hematol Oncol 2009; 27 (02) 102-106
- Austin JH, Müller NL, Friedman PJ. et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996; 200 (02) 327-331
- Tanaka N, Matsumoto T, Miura G. et al. CT findings of leukemic pulmonary infiltration with pathologic correlation. Eur Radiol 2002; 12 (01) 166-174
- Heyneman LE, Johkoh T, Ward S, Honda O, Yoshida S, Müller NL. Pulmonary leukemic infiltrates: high-resolution CT findings in 10 patients. AJR Am J Roentgenol 2000; 174 (02) 517-521
- Reynolds JH, Banerjee AK. Imaging pneumonia in immunocompetent and immunocompromised individuals. Curr Opin Pulm Med 2012; 18 (03) 194-201
- Kisembo HN, Boon SD, Davis JL. et al. Chest radiographic findings of pulmonary tuberculosis in severely immunocompromised patients with the human immunodeficiency virus. Br J Radiol 2012; 85 (1014): e130-e139
- Heussel CP, Kauczor HU, Heussel G, Fischer B, Mildenberger P, Thelen M. Early detection of pneumonia in febrile neutropenic patients: use of thin-section CT. AJR Am J Roentgenol 1997; 169 (05) 1347-1353


 PDF
PDF  Views
Views  Share
Share

