An Assessment of the Three Popular Prognostic Scoring Systems for Chronic Myelomonocytic Leukemia (CMML) in an Indian Context
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(04): 422-427
DOI: DOI: 10.1055/s-0043-1766130
Abstract
Introduction: Chronic myelomonocytic leukemia (CMML) is a rare clonal hematopoietic neoplasm with a prevalence of 1.05 to 1.94 cases per 1,00,000 population. There are multiple prognostic scoring system used in practice for CMML, which include both cytogenetic and next-generation sequencing based.
Objective This study assesses the clinicohematological profile of CMML patients, along with comparison of three widely used prognostic scoring systems for CMML (CMML-specific prognostic scoring system, MD Anderson prognostic score, Mayo prognostic model).
Materials and Methods: This study is an 8-year retrospective study. All relevant data had been retrieved and reviewed by the authors. Inclusion and exclusion criteria: All the cases that were diagnosed before 2016 as per 2008 criteria were reclassified, (2) all the cases that were positive for the mutations associated with myeloproliferative neoplasms were excluded, and (3) cases with more than or equal to 20% blast/blast equivalents were excluded. A univariate analysis was done followed by a multivariate analysis for all the parameters constituting each scoring system. Lastly, a receiver operating characteristic curve was plotted for all the three scoring systems.
Result: There were total 23 patients, with a median age of 63 years and a male to female ratio of 2.3:1. Cytogenetic aberration and genetic mutation were observed in 6 and 3 cases, respectively. The median overall survival (OS) was 48 months and the median leukemia-free survival was 12 months. Post-multivariate analysis, the parameters with significant impact on OS were absolute monocyte count more than 10 × 10^9/L, myeloid precursors in peripheral blood, hemoglobin less than 10g/dL, platelet less than 100 × 10^9/L, hemoglobin less than 12g/dL, and absolute lymphocyte count more than 2.5 × 10^9/L.
Conclusion: To summarize, we discovered CPSS to be a better prognostic tool for a setup like ours, since molecular investigations are not always readily available for each case. More such researches are needed in the near future so that we can design better prognostic tools and see for their usefulness in real life.
Publication History
Article published online:
17 April 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction: Chronic myelomonocytic leukemia (CMML) is a rare clonal hematopoietic neoplasm with a prevalence of 1.05 to 1.94 cases per 1,00,000 population. There are multiple prognostic scoring system used in practice for CMML, which include both cytogenetic and next-generation sequencing based.
Objective This study assesses the clinicohematological profile of CMML patients, along with comparison of three widely used prognostic scoring systems for CMML (CMML-specific prognostic scoring system, MD Anderson prognostic score, Mayo prognostic model).
Materials and Methods: This study is an 8-year retrospective study. All relevant data had been retrieved and reviewed by the authors. Inclusion and exclusion criteria: All the cases that were diagnosed before 2016 as per 2008 criteria were reclassified, (2) all the cases that were positive for the mutations associated with myeloproliferative neoplasms were excluded, and (3) cases with more than or equal to 20% blast/blast equivalents were excluded. A univariate analysis was done followed by a multivariate analysis for all the parameters constituting each scoring system. Lastly, a receiver operating characteristic curve was plotted for all the three scoring systems.
Result: There were total 23 patients, with a median age of 63 years and a male to female ratio of 2.3:1. Cytogenetic aberration and genetic mutation were observed in 6 and 3 cases, respectively. The median overall survival (OS) was 48 months and the median leukemia-free survival was 12 months. Post-multivariate analysis, the parameters with significant impact on OS were absolute monocyte count more than 10 × 10^9/L, myeloid precursors in peripheral blood, hemoglobin less than 10g/dL, platelet less than 100 × 10^9/L, hemoglobin less than 12g/dL, and absolute lymphocyte count more than 2.5 × 10^9/L.
Conclusion: To summarize, we discovered CPSS to be a better prognostic tool for a setup like ours, since molecular investigations are not always readily available for each case. More such researches are needed in the near future so that we can design better prognostic tools and see for their usefulness in real life.
Keywords
CMML - prognostic tools - cytogenetics - overall survival - leukemia-free survival.Introduction
Chronic myelomonocytic leukemia (CMML) is a rare clonal hematopoietic neoplasm with a prevalence of 1.05 to 1.94 cases per 1,00,000 population. The diagnostic criteria for CMML now include both the absolute monocyte count (AMC) and the relative monocyte percentage as part of the criteria.[1]
They can be further subcategorized based on the blast percentage in peripheral blood (PB) and bone marrow (BM) into CMML-0, CMML-1, and CMML-2, as well as based on white blood cell (WBC) count into dysplastic(<13>13 × 10^9/L) types. The proliferative subtype is more commonly seen to be associated with splenomegaly, constitutional symptoms, and JAK2 and RAS mutations, whereas the dysplastic ones are commonly associated with hematopoietic insufficiency symptoms (fatigue, infections, or bleeding).[1] [2]
There are multiple prognostic scoring system used in practice for CMML, such as CMML-specific prognostic scoring system (CPSS), CPSS-molecular (CPSS-Mol), MD Anderson Prognostic Score (MDAPS), Mayo prognostic scoring model, Mayo-molecular model, and Groupe Francophones des Myelodysplasies (GFM). These scoring methods aid in classifying patients into high- and low-risk groups so that a treatment plan may be determined.[3] [4] [5] [6]
This study discusses the clinicopathological profile of CMML patients experienced at our center. We also did a comparison between the three commonly used prognostic scoring systems based on cytogenetics (CPSS, MDAPS, Mayo prognostic model) for CMML patients.
Materials and Methods
This study is an 8-year (72 months) retrospective analysis from January 2013 to December 2021. This study had been conducted in Gujarat Cancer Research Institute, Ahmedabad. All necessary data such as demographics, clinics, laboratory parameters, marrow studies, radiology, cytogenetics, and/or mutation studies, and follow-up had been retrieved from the medical records. Old histopathology and hematology slides were collected and reviewed by the authors. Inclusion and exclusion criteria: (1) all the cases that were diagnosed before 2016 as per 2008 World Health Organization (WHO) classification were reclassified, rest were excluded, (2) all the cases which were positive for the various mutations associated with myeloproliferative neoplasm (MPN) were excluded, and (3) cases with more than 20% blast/blast equivalents were excluded.
Karyotyping and fluorescence in situ hybridization studies were done using phase contrast microscopy. Karyotyping was done using a short-term culture technique and at least 20 metaphases were studied. The cytogenetic risk stratification was done as per the Spanish study by Such et al.[4]
Next-generation sequencing (NGS) data was available in only selected cases (not done in present institute) and it was done primarily on PB. The NGS panel included 40 key DNA targets and 29 driver genes that are known to be associated with major myeloid disorder (including juvenile myelomonocytic leukemia (JMML)).
All the cases were subcategorized according to the WBC counts (dysplastic [<13>13 × 10^9/L]) and blast count (CMML-0,1,2). The CPSS score, MDAPS score, and Mayo prognostic score were calculated for each case. The transfusion requirements were in accordance with the WHO based prognostic scoring system.[7]
Statistical analysis was performed using Statistical Package for the Social Sciences software version 25.0 (SPSS Inc., Chicago, Illinois, United States). A univariate analysis was done using Kaplan–Meier method for the interval from the date of diagnosis till last contact/death (overall survival [OS]) or progression to acute myeloid leukemia (leukemia-free survival [LFS]), to determine a two-tailed p-value for each of the individual parameters of each scoring system. The p-value was considered significant only if less than 0.05. Categorical values were represented as counts and relative frequencies, whereas continuous variables are represented as medians and range. For those parameters with significant p-value on univariate analysis, a multivariate analysis was performed using Cox regression hazard model to assess their independent impact. And lastly a receiver operating characteristic (ROC) curves was plotted for each of the scoring system and the area under the curve was calculated to compare the specificity and sensitivity for each system individually.
Ethics: All the approvals had been taken from the institutional review board. Ethical approval was waived by the local ethics committee of institute in view of the retrospective nature of the study and all the procedures being performed were part of the routine care. All the necessary permission had been taken priorly for collection and analysis of materials and data from the concerned authorities.
Results
Out of the 9,000 cases of hematological malignancies that came to our facility over the past 8 years, we received a total of 23 cases of CMML, with a median age of 63 years (29–76 years) and a predominance of male patients (male to female ratio: 2.3:1). The three scoring systems and all patient characteristics are summarized in [Table 1] along with the risk classification of every case. On marrow examination, we had minimal to nil dysplasia in four cases, while rest had dysplasia in at least one lineage ([Fig. 1]). The cases with minimal to nil dysplasia, however, had a history of persistent monocytosis for more than 3 months or some associated clonal abnormality. Splenomegaly was seen in 10 cases (10/19, 53%), hepatomegaly in 5 (5/19, 27%), and lymphadenopathy in 4 (4/20, 20%). Lactate dehydrogenase (LDH) was elevated in 13/15 (87%) (median LDH: 429/µL). Hepatomegaly, splenomegaly, lymphadenopathy, and LDH levels were not significantly associated with OS or LFS (p-value > 0.05). Cytogenetic aberrations were seen in 6/23 cases (5q deletion with t(4,12)(1), 7q deletion(1), trisomy 8(1), inversion 12(1), inversion Y(1) and complex karyotype(1)). In 3/5 cases, molecular abnormality was seen, one case each of ASXL1, RUNX1, and IDH2 along with NRAS mutation. The case with IDH2 and NRAS mutation also had inversion Y. Among the three cases with molecular abnormality, two had leukemic transformation (ASXL1 and IDH2 with NRAS mutation). In one case out of 23, we also encountered cerebrospinal fluid infiltration.
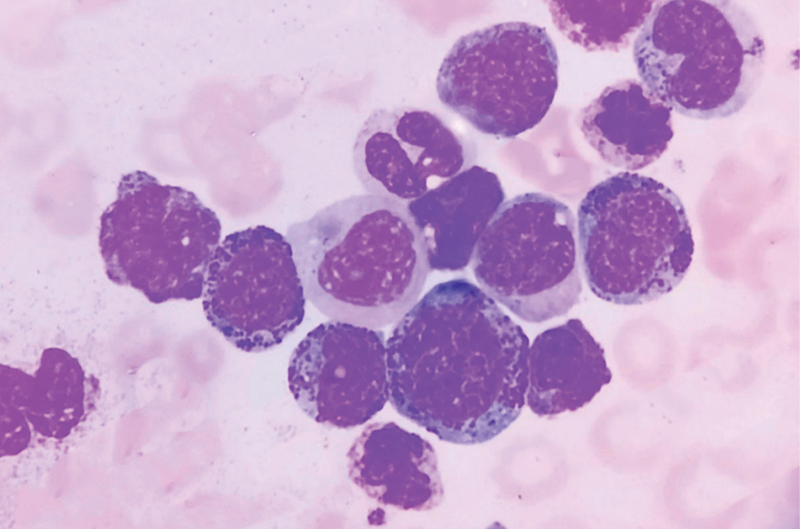
| Figure 1:Peripheral smear of chronic myelomonocytic leukemia, showing proliferation of myeloid and monocytic precursors (1000x oil immersion, Leishman stain).
|
Characteristics |
Median (range) |
Total cases (n = 23) |
|
|---|---|---|---|
|
Age (years) |
63 (29–76) |
||
|
Gender |
Male |
16 (70%) |
|
|
Female |
7 (30%) |
||
|
WHO subtype based on blast% |
CMML-0 |
6 (26%) |
|
|
CMML-1 |
7 (30%) |
||
|
CMML-2 |
10 (43%) |
||
|
FAB subtype based on total leukocyte count |
Dysplastic (<13> |
25.7 (4.8–203) |
5 (22%) |
|
Proliferative (≥13 × 10^9/L) |
18 (78%) |
||
|
Hb (g/dL) |
<10g> |
8.5 (4.5–11.8) |
18 (78%) |
|
≥10g/dL |
5 (22%) |
||
|
<12g> |
23 (100%) |
||
|
≥12g/dL |
0 |
||
|
Platelets (x10^9/L) |
<100> |
90 (7–491) |
13 (57%) |
|
≥100 × 10^9/L |
10 (43%) |
||
|
ALC (x10^9/L) |
>2.5 × 10^9/L |
3.5 (0.54–16.1) |
14 (61%) |
|
≤2.5 × 10^9/L |
9 (39%) |
||
|
AMC (x10^9/L) |
>10 × 10^9/L |
5.47 (1.008–81.2) |
6 (26%) |
|
≤10 × 10^9/L |
17 (74%) |
||
|
Presence of immature myeloid precursors |
Present |
17 (74%) |
|
|
Absent |
6 (26%) |
||
|
Bone marrow blast % |
≥5% |
8 (2–17) |
17 (74%) |
|
<5> |
6 (26%) |
||
|
≥10% |
9 (39%) |
||
|
<10> |
14 (61%) |
||
|
RBC transfusion dependency |
Present |
19 (83%) |
|
|
Absent |
4 (17%) |
||
|
Spanish cytogenetic risk stratification |
Low risk |
15 (66%) |
|
|
Intermediate risk |
4 (17%) |
||
|
High risk |
4 (17%) |
||
|
CPSS score |
Low |
1 (4%) |
|
|
Intermediate 1 |
4 (17%) |
||
|
Intermediate 2 |
14 (61%) |
||
|
High |
4 (17%) |
||
|
MDAPS score |
Low |
3 (13%) |
|
|
Intermediate 1 |
5 (22%) |
||
|
Intermediate 2 |
10 (43%) |
||
|
High |
5 (22%) |
||
|
Mayo clinic score |
Low |
2 (9%) |
|
|
Intermediate |
3 (13%) |
||
|
High |
18 (78%) |
||
|
AML transformation |
4(17%) |
||
|
Expired |
10 (43%) |
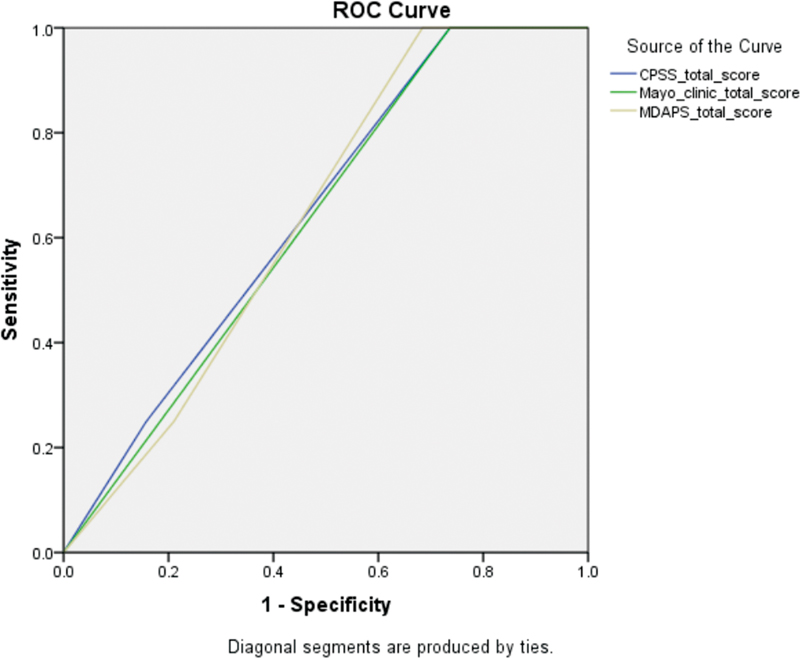
| Figure 2:Receiver operating characteristic (ROC) curve for all the three prognostic tool with area under curve for each. CPSS, chronic myelomonocytic leukemia-specific prognostic scoring system; MDAPS, MD Anderson prognostic score.
|
Characteristics |
n (%) |
OS |
LFS |
||
|---|---|---|---|---|---|
|
Hazard ratio |
Cox regression (p-value) |
Hazard ratio |
Cox regression (p-value)) |
||
|
CPSS score |
|||||
|
BM blast (≥5%) |
17 |
2.64 |
0.056 |
2.43 |
0.017 |
|
WBC≥13 × 10^9/L |
18 |
1.811 |
0.071 |
2.81 |
0.061 |
|
RBC transfusion dependency |
19 |
– |
– |
– |
– |
|
Cytogenetic score |
8 |
– |
– |
– |
– |
|
Mayo clinic score |
|||||
|
AMC >10 × 10^9/L |
6 |
0.266 |
0.012 |
– |
– |
|
IMC in PB |
17 |
0.592 |
0.033 |
1.735 |
0.047 |
|
Hb(<10g> |
17 |
0.572 |
0.031 |
– |
– |
|
Platelet (<100> |
13 |
1.782 |
0.047 |
2.711 |
0.083 |
|
MDAPS score |
|||||
|
Hb(<12g> |
23 |
0.987 |
0.009 |
– |
– |
|
ALC(>2.5 × 10^9/L) |
14 |
0.521 |
0.028 |
– |
– |
|
IMC in PB |
17 |
0.339 |
0.059 |
1.735 |
0.475 |
|
BM blast (≥10%) |
9 |
– |
– |
– |
– |
References
- Orazi A, Benett JM, Germing U. et al. Chronic myelomonocytic leukemia. In: Swerdlow SH, Campo E, Harris NL. et al., eds. WHO Classification of Tumors of Haematopoetic and Lymphoid tissues. 4th ed. Lyon: International agency for research on cancer (IARC); 2017: 82-86
- Patnaik MM, Tefferi A. Chronic myelomonocytic leukemia: 2020 update on diagnosis, risk stratification and management. Am J Hematol 2020; 95 (01) 97-115
- Elena C, Gallì A, Such E. et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood 2016; 128 (10) 1408-1417
- Such E, Cervera J, Costa D. et al. Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica 2011; 96 (03) 375-383
- Onida F, Kantarjian HM, Smith TL. et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood 2002; 99 (03) 840-849
- Patnaik MM, Padron E, LaBorde RR. et al. Mayo prognostic model for WHO-defined chronic myelomonocytic leukemia: ASXL1 and spliceosome component mutations and outcomes. Leukemia 2013; 27 (07) 1504-1510 Erratum in: Leukemia. 2013 Oct;27(10):2112. Komroji, R S [corrected to Komrokji, R S]. PMID: 23531518
- Malcovati L, Germing U, Kuendgen A. et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 2007; 25 (23) 3503-3510
- Guru Murthy GS, Dhakal I, Mehta P. Incidence and survival outcomes of chronic myelomonocytic leukemia in the United States. Leuk Lymphoma 2017; 58 (07) 1648-1654
- Calvo X, Nomdedeu M, Santacruz R. et al. Comparison of three prognostic scoring systems in a series of 146 cases of chronic myelomonocytic leukemia (CMML): MD Anderson prognostic score (MDAPS), CMML-specific prognostic scoring system (CPSS) and Mayo prognostic model. A detailed review of prognostic factors in CMML. Leuk Res 2015; S0145-2126 (15)30324–6 DOI: 10.1016/j.leukres.2015.05.017.
- Hoversten K, Vallapureddy R, Lasho T. et al. Nonhepatosplenic extramedullary manifestations of chronic myelomonocytic leukemia: clinical, molecular and prognostic correlates. Leuk Lymphoma 2018; 59 (12) 2998-3001
- Azeez N, Somasundaram V, Sharma I, Sharma S, Malik A. Clinicopathological profile of chronic myelomonocytic leukemia cases: an experience from a tertiary care center. APLM 2019; 6 (10) 525-530
- Padron E, Garcia-Manero G, Patnaik MM. et al. An international data set for CMML validates prognostic scoring systems and demonstrates a need for novel prognostication strategies. Blood Cancer J 2015; 5 (07) e333 DOI: 10.1038/bcj.2015.53.
- Roman D, Arenillas L, Parraga I. et al. Generation of a new prognostic index for chronic myelomonocytic leukemia (CMML) based on peripheral blood assessment. Blood 2019; 134 (Suppl. 01) 637
- Greenberg PL, Tuechler H, Schanz J. et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012; 120 (12) 2454-2465
- Patnaik MM, Itzykson R, Lasho TL. et al. ASXL1 and SETBP1 mutations and their prognostic contribution in chronic myelomonocytic leukemia: a two-center study of 466 patients. Leukemia 2014; 28 (11) 2206-2212
- Padron E. Chronic myelomonocytic leukemia: Management and prognosis. In: Uptodate 2020. Accessed February 22, 2023 at: http://www.uptodate.com/contents/ChronicmyelomonocyticleukemiaonSept04,2020
Address for correspondence
Jyoti Sawhney, DM Haemato-pathology, Asst. Prof.Department of Oncopathology, Gujarat Cancer Research InstituteAsarwa, Ahmedabad, Gujarat - 380016IndiaEmail: jo_bajaj@yahoo.comPublication History
Article published online:
17 April 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Peripheral smear of chronic myelomonocytic leukemia, showing proliferation of myeloid and monocytic precursors (1000x oil immersion, Leishman stain).

| Figure 2:Receiver operating characteristic (ROC) curve for all the three prognostic tool with area under curve for each. CPSS, chronic myelomonocytic leukemia-specific prognostic scoring system; MDAPS, MD Anderson prognostic score.
References
- Orazi A, Benett JM, Germing U. et al. Chronic myelomonocytic leukemia. In: Swerdlow SH, Campo E, Harris NL. et al., eds. WHO Classification of Tumors of Haematopoetic and Lymphoid tissues. 4th ed. Lyon: International agency for research on cancer (IARC); 2017: 82-86
- Patnaik MM, Tefferi A. Chronic myelomonocytic leukemia: 2020 update on diagnosis, risk stratification and management. Am J Hematol 2020; 95 (01) 97-115
- Elena C, Gallì A, Such E. et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood 2016; 128 (10) 1408-1417
- Such E, Cervera J, Costa D. et al. Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica 2011; 96 (03) 375-383
- Onida F, Kantarjian HM, Smith TL. et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood 2002; 99 (03) 840-849
- Patnaik MM, Padron E, LaBorde RR. et al. Mayo prognostic model for WHO-defined chronic myelomonocytic leukemia: ASXL1 and spliceosome component mutations and outcomes. Leukemia 2013; 27 (07) 1504-1510 Erratum in: Leukemia. 2013 Oct;27(10):2112. Komroji, R S [corrected to Komrokji, R S]. PMID: 23531518
- Malcovati L, Germing U, Kuendgen A. et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 2007; 25 (23) 3503-3510
- Guru Murthy GS, Dhakal I, Mehta P. Incidence and survival outcomes of chronic myelomonocytic leukemia in the United States. Leuk Lymphoma 2017; 58 (07) 1648-1654
- Calvo X, Nomdedeu M, Santacruz R. et al. Comparison of three prognostic scoring systems in a series of 146 cases of chronic myelomonocytic leukemia (CMML): MD Anderson prognostic score (MDAPS), CMML-specific prognostic scoring system (CPSS) and Mayo prognostic model. A detailed review of prognostic factors in CMML. Leuk Res 2015; S0145-2126 (15)30324–6 DOI: 10.1016/j.leukres.2015.05.017.
- Hoversten K, Vallapureddy R, Lasho T. et al. Nonhepatosplenic extramedullary manifestations of chronic myelomonocytic leukemia: clinical, molecular and prognostic correlates. Leuk Lymphoma 2018; 59 (12) 2998-3001
- Azeez N, Somasundaram V, Sharma I, Sharma S, Malik A. Clinicopathological profile of chronic myelomonocytic leukemia cases: an experience from a tertiary care center. APLM 2019; 6 (10) 525-530
- Padron E, Garcia-Manero G, Patnaik MM. et al. An international data set for CMML validates prognostic scoring systems and demonstrates a need for novel prognostication strategies. Blood Cancer J 2015; 5 (07) e333 DOI: 10.1038/bcj.2015.53.
- Roman D, Arenillas L, Parraga I. et al. Generation of a new prognostic index for chronic myelomonocytic leukemia (CMML) based on peripheral blood assessment. Blood 2019; 134 (Suppl. 01) 637
- Greenberg PL, Tuechler H, Schanz J. et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012; 120 (12) 2454-2465
- Patnaik MM, Itzykson R, Lasho TL. et al. ASXL1 and SETBP1 mutations and their prognostic contribution in chronic myelomonocytic leukemia: a two-center study of 466 patients. Leukemia 2014; 28 (11) 2206-2212
- Padron E. Chronic myelomonocytic leukemia: Management and prognosis. In: Uptodate 2020. Accessed February 22, 2023 at: http://www.uptodate.com/contents/ChronicmyelomonocyticleukemiaonSept04,2020


 PDF
PDF  Views
Views  Share
Share

