Affordable and Safe Health Care for All Children: Lessons Learned from the Use of Pegasparaginase in a Developing Country
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 398-400
DOI: DOI: 10.4103/ijmpo.ijmpo_110_17
Abstract
Peg-asparaginase has widely replaced the use of conventional asparaginase in treatment of children with acute lymphoblastic leukaemia in developed countries. In developing countries like India, with financial constraints being a part of clinical challenge to the treatment of cancers, uniform use of Peg-asparaginase in all children is not practically possible. However, we found by a retrospective analysis of 211 children treated for acute lymphoblastic leukaemia, uniform use of this drug was feasible with indigenous techniques like storing the drug with strict cold chain maintenance and sharing the drug amongst 2 or 3 patients to reduce the burden on each family. We have not found increased rates of infection or any loss of efficacy of the drug due to prolonged storage.
Keywords
Acute lymphoblastic leukemia - allergy - fibrinogen - pancreatitis - Peg-asparaginase - thrombosisPublication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Peg-asparaginase has widely replaced the use of conventional asparaginase in treatment of children with acute lymphoblastic leukaemia in developed countries. In developing countries like India, with financial constraints being a part of clinical challenge to the treatment of cancers, uniform use of Peg-asparaginase in all children is not practically possible. However, we found by a retrospective analysis of 211 children treated for acute lymphoblastic leukaemia, uniform use of this drug was feasible with indigenous techniques like storing the drug with strict cold chain maintenance and sharing the drug amongst 2 or 3 patients to reduce the burden on each family. We have not found increased rates of infection or any loss of efficacy of the drug due to prolonged storage.
Pegylated Escherichia coli asparaginase, as part of the medical armamentarium against acute lymphoblastic leukemia (ALL) chemotherapy, has gained importance over the past few years due to its longer half-life, sustained asparagine depletion, effective central nervous system (CNS) protection, and decreased incidence of allergic reactions. However, it is still not being used widely in developing countries as innovation comes at a high cost. For developing countries like India where the children come from lower socioeconomic group with no health insurance, equity of distribution of this drug remains a challenge to the pediatric oncologists. We had developed systematic methods such as sharing the drug and strict cold chain storage up to 28 days to decrease cost of care and demonstrated improved outcomes in our cohort.
We conducted a retrospective analysis of case records of children with ALL between 1 and 18 years who were treated up front at our center from May 2007 when we started using Peg-asparaginase (Oncaspar) with the aim of studying the feasibility of using Peg-asparaginase uniformly, complications of Peg-asparaginase, and outcome in form of event-free survival. Children who had received any therapy elsewhere, children with relapsed ALL, and those lost to follow-up or defaulted treatment were excluded from the analysis.
The UK ALL 2003/2011 protocol was used uniformly. Two doses of 1000 IU/m2 of Peg-asparaginase during induction and one dose during each delayed intensification were used. One vial of Peg-asparaginase having 3750 IU/5 ml was shared among 2 or 3 children. Expenses were proportionately shared among the parents. Each new vial when opened was handled with strict aseptic precautions to prevent bacterial contamination and stored at 4°C for a maximum period of 4 weeks.
Serum fibrinogen was checked at 48 h after Peg-asparaginase injection, and if it was <100>
Of the 211 children who were treated up front at our center, 150 (71.1%) were boys. The median age at presentation was 7.5 years. Based on the risk stratification, 60.2% of children were treated with regimen-A and 39.8% with regimen-B UK ALL protocol. Immunophenotyping revealed 32 T-ALL, 1 Ph + ALL, and 178 B-ALL. One child had CNS disease at diagnosis.
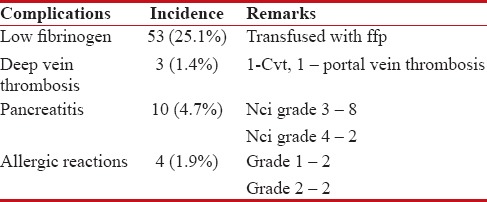
At the end of induction, 25% had MRD positivity and required augmented therapy. The major Peg-asparaginase-associated complications were pancreatitis in 5%, venous thrombosis in 1.4%, and allergic reactions in 2% children. Those who had significant grade 3 or 4 pancreatitis or thrombotic events were not challenged again with the drug. The incidence of venous thrombosis was clearly low at 1.4% as compared to published data from the UK MRC trials at 3.2%.
A total of 211 children have shared 130 vials of Peg-asparaginase. An effective saving of 60% could be made for each child with no major side effects due to prolonged storage.
Event-free survival in our cohort was 88% compared to the UK MRC data. Relapse rate in our cohort was 10.2%, with medullary relapse being the most common. CNS relapse was noted in five children, including three with combined CNS and medullary relapse.
Peg-asparaginase is formed by covalent linking of 5000 dalton units of monomethoxypolyethylene glycol to E. coli asparaginase. Binding preserves the enzyme activity but decreases immunogenicity of the protein. The pharmacokinetic studies have shown slow rise and sustained blood levels of the drug, making it more effective by causing prolonged and sustained asparagine depletion, thereby depriving the leukemic cells of this essential amino acid and causing enhanced cell kill.[1]
Major toxicities associated with asparaginase include hyperglycemia, pancreatitis, allergic reactions, transaminitis, and venous thrombosis. The incidence of each of these in our cohort is tabulated in Table 1. The incidence of allergic reactions was low; however, asparagine antibody level/silent inactivation was not studied in our cohort. Venous thrombosis due to Peg-asparaginase is due to the decreased levels of antithrombin importantly, also contributed by the decreased levels of plasminogen activator. Serum fibrinogen also decreases in parallel with antithrombin level and hence serves as a surrogate marker of coagulopathy.[2,3] In our cohort, we found that 25% children had critically low levels of fibrinogen (<100 xss=removed>P = 0.0017). This could probably be due to the early correction of asymptomatic coagulopathy. Transaminitis was noted in 2.6% children, not requiring treatment interruption. The incidence of pancreatitis is high in our cohort.
Table 1
Toxicity profile of peg.asparaginase in our cohort
| Due to the high cost, it is not a standard practice to use Peg-asparaginase for all children in most of the centers in India. We have developed methods to universalize its use for all children despite the socioeconomic strata. Peg-asparaginase is stored at 2–4°C for maximum period of 4 weeks and handled with strict aseptic precautions. Each vial of 3750 units/mL has been shared among 3–4 children. The cost of each vial was 2200 USD, and if we had used a separate vial for each child, the total amount would have been 671,000 USD. However, 50%–60% savings could be done for each child by sharing the drug. It is the need of the hour to develop indigenous strategies to universalize standard treatment in developing countries with varied socioeconomic distribution of the patient population.[4] We documented an event-free survival of 88%, which is higher than other centers in India with the use of Peg-asparaginase being the backbone of ALL therapy only at our center.
The use of Peg-asparaginase is feasible in children from developing countries despite the social and financial issues. The incidence of deep vein thrombosis can clearly be reduced by monitoring serum fibrinogen. Peg-asparaginase could be safely stored up to 4 weeks and effectively shared to significantly cut down burden on individual family and deliver standard care to all children. The low toxicity profile and excellent clinical efficacy make it the most important way forward to improve outcomes and reduce relapses in our country.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Dinndorf PA1, Gootenberg J, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: Pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). Oncologist 2007;12:991-8.
- Caruso V, Iacoviello L, Di Castelnuovo A, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: A meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood 2006;108:2216-22.
- Hijiyaa N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leukemia and Lymphoma 2016:57:748-57.
- Rowntree C, Hough RE, Wade R, Goulden N, Mitchell C, Vora AJ. Outcomes of teenagers and young adults on the UKALL 2003 paediatric trial for children and young people with acute lymphoblastic leukaemia. Blood 2013;122:57.
References
- Dinndorf PA1, Gootenberg J, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: Pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). Oncologist 2007;12:991-8.
- Caruso V, Iacoviello L, Di Castelnuovo A, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: A meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood 2006;108:2216-22.
- Hijiyaa N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leukemia and Lymphoma 2016:57:748-57.
- Rowntree C, Hough RE, Wade R, Goulden N, Mitchell C, Vora AJ. Outcomes of teenagers and young adults on the UKALL 2003 paediatric trial for children and young people with acute lymphoblastic leukaemia. Blood 2013;122:57.


 PDF
PDF  Views
Views  Share
Share

