Adrenal metastases in a post-radiation malignant fibrous histiocytoma after low-dose radiation for a benign condition
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(01): 31-33
DOI: DOI: 10.4103/0971-5851.113417
Abstract
A 29-year-old male presented with an aggressive malignant fibrous histiocytoma of his leg 14 years after he had received low-dose radiation to the area for a benign indication. The other unusual feature of this case was the large unilateral adrenal metastasis. We describe this very rare presentation of sarcoma and briefly review the relevant literature.
Publication History
Article published online:
20 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
A 29-year-old male presented with an aggressive malignant fibrous histiocytoma of his leg 14 years after he had received low-dose radiation to the area for a benign indication. The other unusual feature of this case was the large unilateral adrenal metastasis. We describe this very rare presentation of sarcoma and briefly review the relevant literature.
INTRODUCTION
Post-radiation sarcoma (PRS) is a well-described entity, constituting 2-3% of all sarcomas.[1,2,3] It occurs after 4-55 years in 0.6-0.8% patients who receive radiation for various indications.[2,3] Often, they are aggressive tumors; metastases are common and they have a poor prognosis.[1,2,3,4] Osteosarcoma is the most commonly occurring PRS overall, but among soft tissue tumors, malignant fibrous histiocytoma (MFH) predominates.[1,2,3] We describe a patient who developed post-radiation MFH with a few unusual features.
CASE REPORT
A 29-year-old male noted right leg swelling which rapidly grew over 4 months. Simultaneously, he developed anorexia and pain in his abdomen. At presentation, he had a soft tissue tumor involving the entire right leg with skin infiltration and ulceration [Figure 1a]. He also had a large right hypochondrial abdominal mass. Details of past history revealed that 15 years back he had had a local resection of a hemangioma in his right leg. This lesion had recurred a year later which had been treated with local radiotherapy (20 Gy in 5 fractions). Thereafter, he had remained asymptomatic till the current presentation.
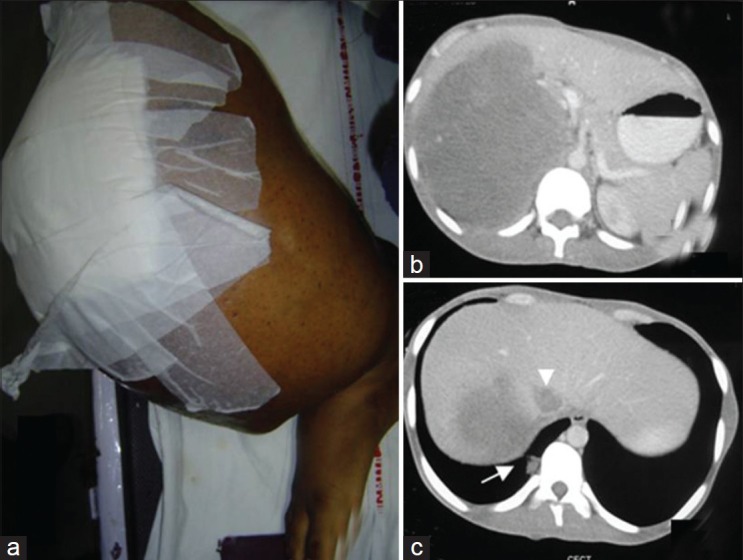
| Fig. 1 A large tumor mass in the right leg with skin involvement and ulceration (wound dressing done) (a) CT scan of the abdomen shows a large adrenal lesion (b) with infiltration of the liver (c) inferior Vena Caval thrombus (c, arrowhead), and lung metastasis (c, arrow)
Magnetic resonance imaging showed a large soft tissue mass with heterogeneous signal intensity (areas suggestive of hemorrhage and necrosis) involving the entire right leg including the tibial and fibular diaphysis. Abdominal computerized tomography (CT) scan showed a right suprarenal lesion with mass effect on the kidney and liver infiltration [Figure 1b]; additionally inferior vena cava thrombosis was also noted [Figure 1c]. Bone scan showed lesions only in the right tibia and fibula; chest CT scan showed lung metastases [Figure 1c]. In view of the very large ulcerating tumor, the patient underwent a palliative right above knee amputation. Histopathology of the resected specimen showed an MFH with storiform pattern of growth and 5-6 mitoses per high power field; the cells were highly pleomorphic with presence of tumor giant cells, areas of necrosis, and atypical mitosis; histiocytic nature of the tumor was confirmed by CD68 positivity [Figure [Figure2a2a–d]. Evaluation for a primary adrenal disease revealed normal serum cortisol and urinary catecholamines. Biopsy from the adrenal mass showed a metastatic tumor morphologically similar to the primary leg sarcoma. The patient was started on palliative chemotherapy with single agent doxorubicin; he, however, discontinued after 2 cycles of chemotherapy due to progressive disease.
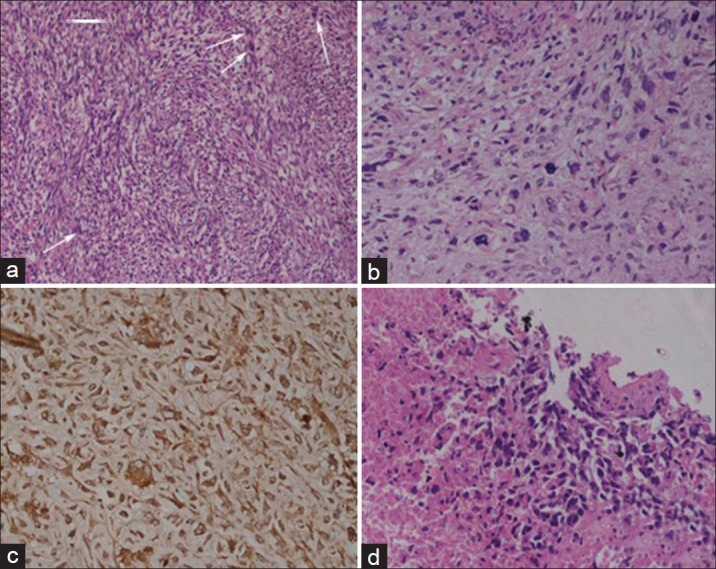
| Fig. 2 Histology of amputated specimen shows sarcoma with storiform pattern and mitotic figures (arrows) (H and E, ×200) (a) highly pleomorphic tumor cells and atypical mitosis (H and E, ×400) (b) and immunohistochemical positivity for CD68 (Immunoperoxidase, ×200) (c) biopsy from adrenal lesion showing necrosis and metastatic sarcoma with similar morphology (H and E, ×400) (d)
DISCUSSION
Ionizing radiation has been shown to cause genetic defects including defects in p53 expression which may predispose to cancer.[5] Though the entity of PRS had been described earlier, it gained acceptance only in 1948, when Cahan et al. proposed criteria for diagnosing PRSs of the bone.[6] In our patient, the prior history of radiation, the long latency from the original radiation, and the development of histologically confirmed sarcoma in the previously irradiated field all point toward a diagnosis of PRS.
Our patient had received a relatively small dose of 20 Gy which had caused the development of sarcoma 14 years later. PRS can occur with both low- and high-energy radiation though, and a well-defined threshold dose does not exist.[6] Most of the earlier series described cases who had received a median of 50-60 Gy and it was doubted whether low-dose radiation could actually cause sarcoma.[7] In a series of 47 cases described in 1978, none occurred with doses less than 30 Gy.[8] Recent reports, however, have described sarcomas developing even with low doses of radiation.[9] In a series of 65 patients of PRS, Strauss et al. reported four cases who had received ≤20 Gy radiation.[5]
Our patient had abdominal pain as a predominant presenting feature, and had a large abdominal lump. Adrenal metastasis, though described in sarcomas including MFH,[10] is very uncommon and even rarely diagnosed clinically. Most of the described cases have been seen postmortem.[11,12] In a series of 421 metastatic adrenal tumors, only 7 cases were from sarcomas, all of which were detected post mortem.[13] In the report by Kobayashi et al., antemortem diagnosis of adrenal metastasis in an asymptomatic patient was possible only by using (F-18)Fluorine 18 Flurodeoxyglucose Positron Emission Tomography-CT imaging.[10] To our knowledge, this is the first report of a PRS presenting with adrenal metastasis.
The entity of PRS is a disastrous outcome of potentially curative therapies for many tumors. Current radiation practices focus on limiting both the field and dose of radiation to reduce the risks of PRS. Unfortunately, our patient received radiation for a benign indication which led to disastrous consequences much later in life. The case evidences the need for prolonged follow-up in patients who have been treated with ionizing radiation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.

| Fig. 1 A large tumor mass in the right leg with skin involvement and ulceration (wound dressing done) (a) CT scan of the abdomen shows a large adrenal lesion (b) with infiltration of the liver (c) inferior Vena Caval thrombus (c, arrowhead), and lung metastasis (c, arrow)

| Fig. 2 Histology of amputated specimen shows sarcoma with storiform pattern and mitotic figures (arrows) (H and E, ×200) (a) highly pleomorphic tumor cells and atypical mitosis (H and E, ×400) (b) and immunohistochemical positivity for CD68 (Immunoperoxidase, ×200) (c) biopsy from adrenal lesion showing necrosis and metastatic sarcoma with similar morphology (H and E, ×400) (d)


 PDF
PDF  Views
Views  Share
Share

