Adrenal Mass: Unusual Presentation and Outcome
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 256-260
DOI: DOI: 10.4103/ijmpo.ijmpo_33_16
Abstract
Aim: Adrenal mass may be functioning or nonfunctioning with varied clinical presentations. This study aimed to report the nature and management of uncommon adrenal mass and to review literature. Materials and Methods: This was an retrospective observational analysis of children with uncommon adrenal mass admitted during 2009–2015. Clinical features, investigations, and management of patients were analyzed. Results: Among six, two each were adolescent and neonate, and one each was young infant and prenatal. Clinical presentation was variable; hypertensive retinopathy,[1] virilization[1] and bleeding diathesis,[1] antenatal suprarenal mass,[1] prenatal adrenal angiolipoma,[1] and spontaneous resolution of Stage III suprarenal mass.[1] Ultrasound and contrast-enhanced computed tomography revealed well-defined, heterogeneous adrenal mass. Size varied from 2 to 15 cm. Urinary metanephrine and serum testosterone were raised in adolescent hypertensive boys and virilized girls, respectively. Laparoscopy-assisted adrenalectomy was done in two and other four were managed conservatively. Histopathology of tumor revealed pheochromocytoma and borderline oncocytoma. Spontaneous resolution of adrenal mass had varied etiology; adrenal hemorrhagic lesion,[1] simple cyst,[1] neuroblastoma.[1] Follow-up varied from 3 months to 2 years. All patients were asymptomatic on last follow-up. Conclusion: Close clinical follow-up, contrast-enhanced tomography, and limited/specific endocrine work-up have definite role in the management of uncommon adrenal mass.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Aim:
Adrenal mass may be functioning or nonfunctioning with varied clinical presentations. This study aimed to report the nature and management of uncommon adrenal mass and to review literature.
Materials and Methods:
This was an retrospective observational analysis of children with uncommon adrenal mass admitted during 2009–2015. Clinical features, investigations, and management of patients were analyzed.
Results:
Among six, two each were adolescent and neonate, and one each was young infant and prenatal. Clinical presentation was variable; hypertensive retinopathy,[1] virilization[1] and bleeding diathesis,[1] antenatal suprarenal mass,[1] prenatal adrenal angiolipoma,[1] and spontaneous resolution of Stage III suprarenal mass.[1] Ultrasound and contrast-enhanced computed tomography revealed well-defined, heterogeneous adrenal mass. Size varied from 2 to 15 cm. Urinary metanephrine and serum testosterone were raised in adolescent hypertensive boys and virilized girls, respectively. Laparoscopy-assisted adrenalectomy was done in two and other four were managed conservatively. Histopathology of tumor revealed pheochromocytoma and borderline oncocytoma. Spontaneous resolution of adrenal mass had varied etiology; adrenal hemorrhagic lesion,[1] simple cyst,[1] neuroblastoma.[1] Follow-up varied from 3 months to 2 years. All patients were asymptomatic on last follow-up.
Conclusion:
Close clinical follow-up, contrast-enhanced tomography, and limited/specific endocrine work-up have definite role in the management of uncommon adrenal mass.
Introduction
Adrenal tumors may be functioning or nonfunctioning, and clinical presentations depend on the type of hormone production. Functioning type includes virilization, cushing's syndrome, feminization features, and malignant hypertensive retinopathy.[1] Close clinical follow-up, endocrine work-up, and contrast-enhanced computed tomography (CECT) are helpful in assessing the adrenal mass and optimizing the success of adrenalectomy.[1,2,3] Spontaneous resolution of variable adrenal mass will determine the management approach. Unilateral uncommon adrenal mass includes virilized oncocytoma, neonatal spontaneous regression of neuroblastoma, hemorrhage, and cyst.
We report varied adrenal masses with unusual clinical presentations, etiopathogenesis, and successes of the close clinical follow-up.
Materials and Methods
This was an retrospective observation analysis of six adrenal masses admitted in pediatric surgery department during 2009–2015. Literature was reviewed with respect to uncommon modes of presentation and their management.
Results
Case series of uncommon adrenal mass [Table 1].
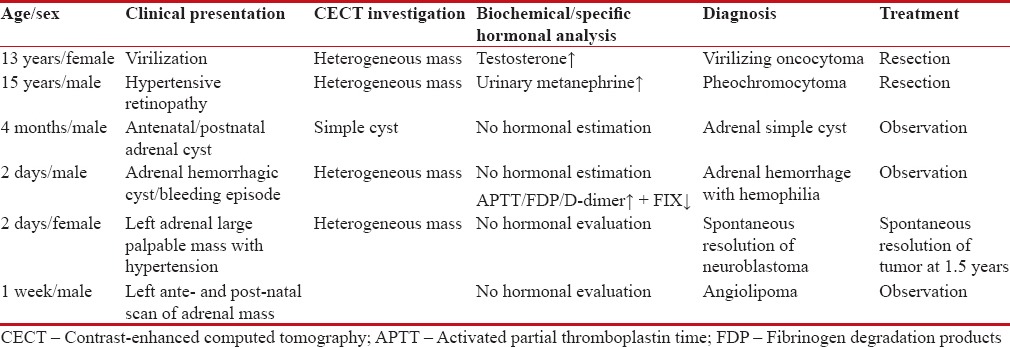
Table 1
Clinical profile of adrenal mass
|
Case report 1
An 11-year-old girl was referred to us with a history of hirsutism and left adrenal mass measuring 6 cm × 5 cm × 4.3 cm with calcification on ultrasound and CECT [Figure 1]. She had enlarged clitoris since birth and was not evaluated completely. Her weight was 30 kg. Vital parameters were normal. General physical and genital examinations showed acne, generalized hirsutism, clitoromegally, normal vagina and urethra, and tanner's Stage 2 of breast and Stage 5 of pubic hair. Terminal hairs were seen on upper lip, chin, lower abdomen, and thigh. Modified Ferriman and Gallwey score (mFG) was 13. Ambo-sexual and sexual hair were prominent. Abdominal mass was nonpalpable. Systemic examinations were normal. Renal function test and serum electrolytes were normal. Limited endocrine work-up was done due to financial constraints which revealed normal serum 17a-hydroxyprogesterone (2.4 ng/ml), testosterone (2.02 ng/ml), and thyroid-stimulating hormone (0.89 mIU/L). She underwent open adrenalectomy after bleeding episode during the laparoscopic dissection of adrenal mass and also had bilateral gonadal biopsy. Histopathology revealed oncocytoma with borderline malignancy which include mitotic score (3/50 high-power field), focal necrosis, calcification, no capsular or vascular invasion [Figure 2], and normal gonads. She is on regular follow-up for 3 years without any complications or recurrence. She started showing signs of feminizations 6 months after surgery including attainment of menarche with regular cycles, disappearance of adrenarche or mFG score reduction to 3, and tanner 4 stage breast development.
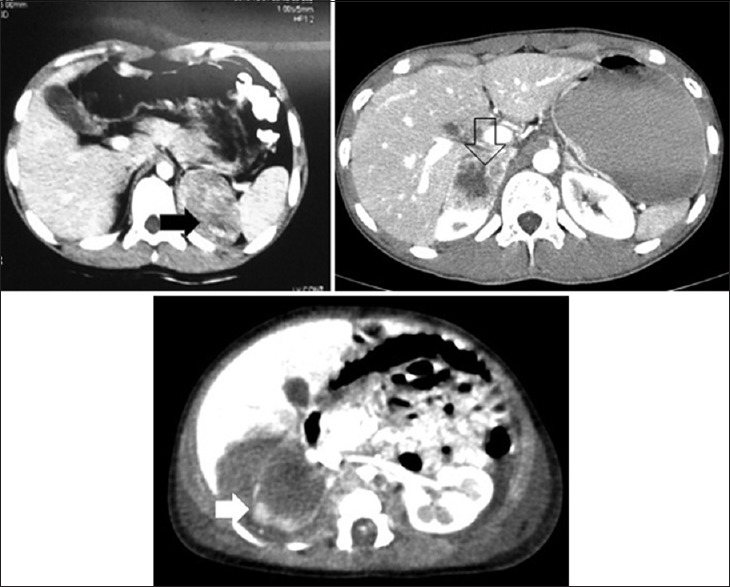
| Figure 1:Contrast-enhanced computed tomography scan showing various adrenal masses (black arrow) oncocytoma, (empty arrow) pheochromocytoma, (white arrow) adrenal hemorrhage

| Figure 2:Diffuse sheets of large polyhedral cells with abundant granular eosinophilic cytoplasm (solid arrows) and necrosis (hallow arrow) suggestive of borderline oncocytoma
Case report 2
A 14-year-old boy presented with giddiness and blurring of vision due to hypertensive Grade IV retinopathy. CECT and ultrasound with Doppler revealed heterogeneous, well-defined adrenal mass measuring 5 cm × 6 cm × 4 cm [Figure 1]. Family history was noncontributory. Echocardiography showed mild left ventricular hypertrophy. Urinary metanephrine was high (19,000). Liver function test and serum electrolytes were normal. He underwent laparoscopy-assisted adrenalectomy after control of hypertension with three drugs. Postoperatively, he had persistent hypotension with normal heart rate and responded to fluid challenge and colloids. Histology of tumor revealed benign pheochromocytoma. All anti-hypertensive drugs were weaned off within 3 weeks and vision had improved. He is asymptomatic for 1 year.
Case report 3
A 2-day-old boy presented with hematoma of the right inguinoscrotal region. Ultrasound and CECT revealed well-defined mixed adrenal mass [Figure 1] pleural effusion, and hydrocele with abdominal wall edema. Prothrombin time, international normalized ratio, activated partial thromboplastin time, fibrinogen degradation products, and D-dimer were raised. He required fresh frozen plasma, cryoprecipitate, and packed red blood cell. Clinically, groin hematoma, hydrocele, and adrenal mass on ultrasound were regressed completely on 2 and 4 weeks of life.
Case report 4
A 4-month-old boy presented with ante- and post-natal abdominal scan of the left cystic adrenal mass. CECT revealed adrenal cystic mass. He was under regular sonological follow-up which showed complete regression without causing any symptoms.
Case report 5
A 2-day-old girl presented with antenatal solid abdominal mass, and abdomen examination detected a large, firm abdominal mass occupying the left half of the abdomen. Ultrasound and CECT scan of the abdomen revealed a large, solid suprarenal mass [Figure 3] crossing midline pushing the surrounding structures peripherally. Her hypertension was controlled with single anti-hypertensive drug. She was planned for fine-needle aspiration cytology (FNAC) but she went against medical advice. She came to us after 1.5 years with scaphoid abdomen and CECT scan revealed spontaneous mass regression [Figure 3]. At last follow-up, she was asymptomatic.
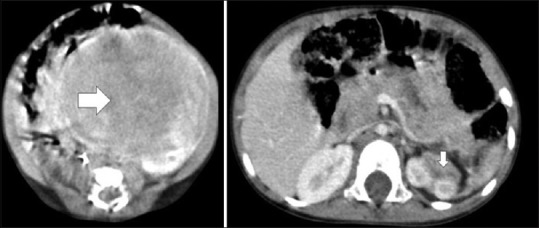
| Figure 3:Contrast-enhanced computed tomography showing spontaneous resolution of left suprarenal mass (horizontal arrow) at 1st week of life and (vertical arrow) at 1.5 years
Case report 6
An neonate presented with ante- and post-natal abdominal scans of heterogeneous and hyperechoic, left, nonprogressive adrenal mass measuring 2 cm, suggestive of angiolipoma. He was under close observation.
Discussion
Common virilizing presentations in adolescent girls are frontal hair thinning, moustache, beard, acne, voice change, enlarged clitoris, poorly developed breast, menstrual problems, accelerated skeletal growth, and maturation.[2] The probable causes of virilization are overproduction of androgens from ovary, adrenals, and pituitary gland, congenital adrenal hyperplasia (CAH), excessive intake of anabolic steroids, hyperandrogenic-insulin-resistant acanthosis nigricans syndrome, intracranial lesions of hypothalamus, and idiopathy.[2,3] Adrenarche may start as early as at 6 years of life. Vellus and terminal hair growth depends on thyroid hormones, androgen levels, and the body growth.[4] Frank hirsutism patients will have excess androgens ranging 80%–90%. Terminal hair growth on the upper lip, chin, and lower abdomen is pathological. Its size is usually >5 mm, coarse, medullated, and pigmented.[4] mFG score can be used for the quantification of hirsutism with photography, visual scoring, and assessing the therapeutic response to tumor excision.[4] There are nine different hormone-sensitive areas and each area can be scored from 0 to 4. Hirsutism is defined if mFG score is >2–3[4] in Asian population. The differential diagnoses of unilateral adrenal mass in adolescent girls are cortical adenoma, carcinoma, metastasis, oncocytoma, pheochromocytoma, and neuroblastoma.[5,6,7,8]
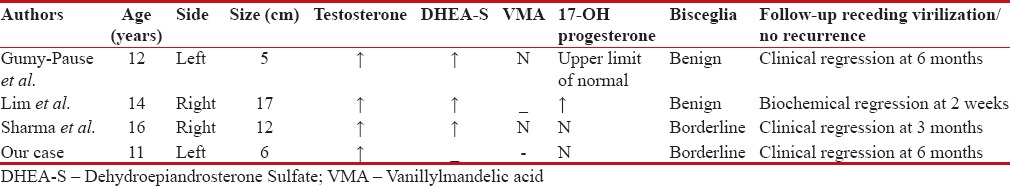
Virilizing adrenal cortical oncocytoma is rare in children.[2] Only two borderline cases were reported till now [Table 2].[2,5,7] It is common in females and predominantly occurs on left adrenal and has better prognosis than nonfunctioning oncocytoma. Estimation of plasma adrenal androgens, testosterone, 17-α hydroxyprogesterone, and 24-h urinary excretion of 17-ketosteroids will ultimately confirm the clinical diagnosis of adrenally induced virilization.[1,9,10,11] CECT scan of benign adrenal oncocytoma usually have well-defined capsule and homogeneous density.[5,7] Tumor cells are polygonal epithelial cells and have intensely eosinophilic granular cytoplasm with packed mitochondria.[3] Oncocytoma is a mitochondrial tumor.[10] Benign or malignancy depends on clinical, biochemical, and histological analyses.[3] Bisceglia proposed malignancy scoring system based on histology.[5,6,7,8] Adrenocorticotropic hormone surge and development of functional adrenal oncocytoma might have caused early adrenarche in our case.[9] “Cytochrome P450c17, P450 oxidoreductase and cytochrome b5 complex, 17, 20-lyase, and backdoor pathway” to dihydrotestosterone factors will determine adrenal virilization.[12] Idiopathic neonatal congenital clitoromegally needs close observation after excluding CAH and maternal factors. Testosterone and androstenedione are interconvertible. Preoperative testosterone/adrenal androgens and 17-α hydroxyprogesterone estimations are sufficient in young adolescent virilize adrenal mass in a girl of being normotensive and having normal electrolytes, before embarking adrenalectomy[1,2] [Table 2]. There are no clear-cut chemoradiotherapy guidelines for borderline malignancy as there were only few case reports of these diseases.[2,5,10] Appearance and persistence of feminization as early as 3 months are good clinical signs of successful tumor excision [Table 2]. Disease-free 5-year survival is considered the cure.
Table 2
Literature review of clinical details of virilizing adrenal oncocytoma and follow-up
|
Immunohistochemistry (IHC) of antimitochondrial antibody, melan-A, synaptophysin, vimentin, and keratin are positive in oncocytoma.[5,6,10] Histology and IHC are the confirmative evidences of oncocytoma and may have prognostic value.[7,10] Indications for adrenalectomy are functioning tumor and size more than 6 cm.[3,10]
Antenatal/postnatal adrenal hemorrhage and neuroblastoma mimic each other both clinically and radiologically.[11,13] Adrenal hemorrhage may have varied etiology including birth trauma, asphyxia, sepsis, disseminated intravascular coagulation (DIC), and hemophilia. Neonatal hemophilia diagnosis was difficult in our case because of negative family history, immature neonatal hematological system, and having presentations of combined coagulopathy due to DIC and sepsis. Close follow-up showed the regression in hemorrhagic lesions, and follow-up hematological work-up had made the diagnosis of hemophilia easy in our case. Hemophilia should be suspected as one of the causes for such extensive/multiple bleeding lesions. Till date, only thirty cases of neonatal adrenal hemorrhage with scrotal hematoma were reported.[14] Clot waveform analysis method is an ideal investigation of choice in the management of complex coagulopathy.
Urinary/plasma metanephrine evaluation is a highly accurate and cost-effective screening test for adolescent boys with resistant hypertensive retinopathy and adrenal incidentaloma.[15] Adrenal pheochromocytoma secretes commonly epinephrine and may present sustained or paroxysmal hypertension. FNAC should be avoided in suspected pheochromocytoma cases where the resection is curative and diagnostic.[16] Ventricle hypertrophy is due to overproduction of collagen and fibronectin.[17] Intraoperative hypovolemia, sudden cessation of catecholamine secretions, persistence of inhibited central sympathetic neural outflow and augmented parasympathetic nerve activity, and half-life of phenoxybenzamine have been blamed for postoperative hypotension;[17] however, later, three factors were predominant in our case causing hypotension with normal heart rate. Hypertensive Grade IV retinopathy and vision had improved after tumor excision which had made the prognostic implication that even higher grade retinopathy could be reversible. Genetic testing for adrenal benign/sporadic pheochromocytoma is optional. Malignancy may be suspected if CECT shows increase in size, calcification, enhancement, and necrosis.[15,16] Infantile adrenal cysts can be pseudocyst, epithelial, and endothelial and are usually the result of developmental malformation or hemorrhagic episode. Antenatal angiolipoma or cysts should be managed conservatively till they become symptomatic or biochemically active. Specific[2] or no hormonal evaluation[4] protocol was successful in our short case series.
Common causes for conversion to open adrenalectomy are bleeding, oncological issues, and learning curve of laparoscopic adrenalectomy (initial 20–40 cases).[18] Oncological issues, abnormal renal vessels, and learning curve had made the decision to perform open adrenalectomy in our cases. Inhibition of the tropomyosin receptor kinase A pathway, depriving cells with nerve growth factors, cellular or humoral immune responses, telomere shortening, or epigenetic modifications might have accelerated the apoptosis of neuroblastoma.[19] The incidence peak of antenatal neuroblastoma is around 16–20 weeks. Literature review had reports of spontaneous resolution or maturation to benign lesion. Though neonates' spontaneous regression of Stage III neuroblastoma is an uncommon and surprise event, these neonates can be observed for a brief period before embarking neoadjuvant chemotherapy and surgical procedure.
Although our case series is a retrospective study, it has emphasized the role of close clinical follow-up and contributed knowledge toward the natural resolution of some uncommon adrenal masses which is worth of reporting to the attention of relevant clinicians.
Conclusion
Adrenal virilizing oncocytoma is a rare tumor and needs limited endocrinologist work-up before embarking on adrenalectomy. Feminization signs and mFG score downgrading are good clinical prognostic indicators. Close clinical and radiological follow-up is required in borderline malignancy and to differentiate benign hemorrhagic cyst from neuroblastoma in neonates. Hypertensive retinopathy could be the first sign of pheochromocytoma, especially in children, and could be reversible completely. Neonatal Stage III neuroblastoma can be observed for a brief period before aggressive management. Close clinical follow-up may have successful outcome in some uncommon adrenal masses.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Nasir J, Walton C. Adrenal mass with virilisation: Importance of endocrine investigation. BMJ 1996;313:872-3.
- Lim YJ, Lee SM, Shin JH, Koh HC, Lee YH. Virilizing adrenocortical oncocytoma in a child: A case report. J Korean Med Sci 2010;25:1077-9
- Tahar GT, Nejib KN, Sadok SS, Rachid LM. Adrenocortical oncocytoma: A case report and review of literature. J Pediatr Surg 2008;43:E1-3.
- Yildiz BO, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update 2010;16:51-64.
- Sharma D, Sharma S, Jhobta A, Sood RG. Virilizing adrenal oncocytoma. J Clin Imaging Sci 2012;2:76.
- Sahin SB, Yucel AF, Bedir R, Ogullar S, Ayaz T, Algun E. Testosterone-and cortisol-secreting adrenocortical oncocytoma: An unusual cause of hirsutism. Case Rep Endocrinol 2014;2014:206890.
- Yoon JH, Cha SS, Yoon SK. Computed tomography and magnetic resonance images of adrenocortical oncocytoma cases. J Korean Med Sci 2014;29:445-51.
- Gumy-Pause F, Bongiovanni M, Wildhaber B, Jenkins JJ, Chardot C, Ozsahin H. Adrenocortical oncocytoma in a child. Pediatr Blood Cancer 2008;50:718-21.
- Mills IH. Virilizing syndromes. Postgrad Med J 1960;36:176-85.
- Mearini L, Del Sordo R, Costantini E, Nunzi E, Porena M. Adrenal oncocytic neoplasm: A systematic review. Urol Int 2013;91:125-33.
- Eo H, Kim JH, Jang KM, Yoo SY, Lim GY, Kim MJ, et al. Comparison of clinic radiological features between congenital cystic neuroblastoma and neonatal adrenal hemorrhagic pseudocyst. Korean J Radiol 2011;12:52-8.
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011;32:81-151.
- McHugh K. Renal and adrenal tumours in children. Cancer Imaging 2007;7:41-51.
- ;Lai LJ, Chen LM, Chu PY, Tseng MH, Chang CC, Lu CW. Neonatal adrenal hemorrhage associated with scrotal hematoma: An unusual case report and literature review. Pediatr Neonatol 2012;53:210-2.
- Schwartz GL. Screening for adrenal-endocrine hypertension: Overview of accuracy and cost-effectiveness. Endocrinol Metab Clin North Am 2011;40:279-94, vii.
- Hanna S, El-Kalioubie M, Badawy H, Halim M. Optimal diagnosis of adrenal masses. Egypt J Radiol Nucl Med 2015;46:511.
- Zuber SM, Kantorovich V, Pacak K. Hypertension in pheochromocytoma: Characteristics and treatment. Endocrinol Metab Clin North Am 2011;40:295-311, vii.
- Pedziwiatr M, Wierdak M, Ostachowski M, Natkaniec M, Bialas M, Hubalewska-Dydejczyk A, et al. Single center outcomes of laparoscopic transperitoneal lateral adrenalectomy – Lessons learned after 500 cases: A retrospective cohort study. Int J Surg 2015;20:88-94.
- Brodeur GM, Bagatell R. Mechanisms of neuroblastoma regression. Nat Rev Clin Oncol 2014;11:704-13.

| Figure 1:Contrast-enhanced computed tomography scan showing various adrenal masses (black arrow) oncocytoma, (empty arrow) pheochromocytoma, (white arrow) adrenal hemorrhage

| Figure 2:Diffuse sheets of large polyhedral cells with abundant granular eosinophilic cytoplasm (solid arrows) and necrosis (hallow arrow) suggestive of borderline oncocytoma

| Figure 3:Contrast-enhanced computed tomography showing spontaneous resolution of left suprarenal mass (horizontal arrow) at 1st week of life and (vertical arrow) at 1.5 years
References
- Nasir J, Walton C. Adrenal mass with virilisation: Importance of endocrine investigation. BMJ 1996;313:872-3.
- Lim YJ, Lee SM, Shin JH, Koh HC, Lee YH. Virilizing adrenocortical oncocytoma in a child: A case report. J Korean Med Sci 2010;25:1077-9
- Tahar GT, Nejib KN, Sadok SS, Rachid LM. Adrenocortical oncocytoma: A case report and review of literature. J Pediatr Surg 2008;43:E1-3.
- Yildiz BO, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update 2010;16:51-64.
- Sharma D, Sharma S, Jhobta A, Sood RG. Virilizing adrenal oncocytoma. J Clin Imaging Sci 2012;2:76.
- Sahin SB, Yucel AF, Bedir R, Ogullar S, Ayaz T, Algun E. Testosterone-and cortisol-secreting adrenocortical oncocytoma: An unusual cause of hirsutism. Case Rep Endocrinol 2014;2014:206890.
- Yoon JH, Cha SS, Yoon SK. Computed tomography and magnetic resonance images of adrenocortical oncocytoma cases. J Korean Med Sci 2014;29:445-51.
- Gumy-Pause F, Bongiovanni M, Wildhaber B, Jenkins JJ, Chardot C, Ozsahin H. Adrenocortical oncocytoma in a child. Pediatr Blood Cancer 2008;50:718-21.
- Mills IH. Virilizing syndromes. Postgrad Med J 1960;36:176-85.
- Mearini L, Del Sordo R, Costantini E, Nunzi E, Porena M. Adrenal oncocytic neoplasm: A systematic review. Urol Int 2013;91:125-33.
- Eo H, Kim JH, Jang KM, Yoo SY, Lim GY, Kim MJ, et al. Comparison of clinic radiological features between congenital cystic neuroblastoma and neonatal adrenal hemorrhagic pseudocyst. Korean J Radiol 2011;12:52-8.
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011;32:81-151.
- McHugh K. Renal and adrenal tumours in children. Cancer Imaging 2007;7:41-51.
- ;Lai LJ, Chen LM, Chu PY, Tseng MH, Chang CC, Lu CW. Neonatal adrenal hemorrhage associated with scrotal hematoma: An unusual case report and literature review. Pediatr Neonatol 2012;53:210-2.
- Schwartz GL. Screening for adrenal-endocrine hypertension: Overview of accuracy and cost-effectiveness. Endocrinol Metab Clin North Am 2011;40:279-94, vii.
- Hanna S, El-Kalioubie M, Badawy H, Halim M. Optimal diagnosis of adrenal masses. Egypt J Radiol Nucl Med 2015;46:511.
- Zuber SM, Kantorovich V, Pacak K. Hypertension in pheochromocytoma: Characteristics and treatment. Endocrinol Metab Clin North Am 2011;40:295-311, vii.
- Pedziwiatr M, Wierdak M, Ostachowski M, Natkaniec M, Bialas M, Hubalewska-Dydejczyk A, et al. Single center outcomes of laparoscopic transperitoneal lateral adrenalectomy – Lessons learned after 500 cases: A retrospective cohort study. Int J Surg 2015;20:88-94.
- Brodeur GM, Bagatell R. Mechanisms of neuroblastoma regression. Nat Rev Clin Oncol 2014;11:704-13.


 PDF
PDF  Views
Views  Share
Share

