Adjuvant therapy for resected high-risk colon cancer: Current standards and controversies
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(03): 197-202
DOI: DOI: 10.4103/0971-5851.142032
Abstract
This evidence-based review will discuss the current standard of adjuvant chemotherapy for resected high-risk colon cancer and address existing controversies including strategies for risk-stratification, the status of targeted therapy, treatment of the elderly and the optimal duration of therapy.
Publication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
This evidence-based review will discuss the current standard of adjuvant chemotherapy for resected high-risk colon cancer and address existing controversies including strategies for risk-stratification, the status of targeted therapy, treatment of the elderly and the optimal duration of therapy.
INTRODUCTION
Colorectal cancer (CRC) is the third most frequent cancer diagnosed worldwide, with nearly 1.4 million new cases diagnosed in 2012.[1] Approximately 75% of these patients are diagnosed with early stage disease (stages I-III) who may be cured with surgery alone or with the addition of adjuvant chemotherapy, as in high-risk stage II and stage III disease. In view of the high-burden of early stage disease, the role of adjuvant chemotherapy has evolved over the last two decades and has resulted in significant improvements in both disease free survival (DFS) and overall survival (OS).
This review will discuss the current standard of adjuvant chemotherapy for resected high-risk colon cancer and address existing controversies including strategies for risk-stratification, the status of targeted therapy, treatment of the elderly and the optimal duration of therapy.
EVOLUTION OF ADJUVANT CHEMOTHERAPY FOR HIGH-RISK COLON CANCER
As summarized in Table 1, a number of randomized phase III trials in the past two decades have established the benefit of 5-fluorouracil (5-FU) in the management of resected stage II/III colon cancer, as well as the current accepted duration of therapy of 24 weeks. In 2004, we reported an individual patient-data pooled analysis of over 3000 patients from seven randomized clinical trials with stage II/III colon cancer.[6] The use of adjuvant 5-FU-based chemotherapy was associated with 30% proportional reduction in risk of recurrence (hazard ratio [HR]: 0.70; range: 0.63-0.78). The Adjuvant Colon Cancer End Points (ACCENT) represents the largest pooled analyses of the individual patient-data in this setting with 20,898 patients in 18 randomized clinical trials. The ACCENT analysis showed that there was a 10% OS benefit of surgery versus 5-FU/LV in stage III. Moreover, most relapses occur within 2 years after surgery. An update by Sargent et al. suggested that in all patients, the DFS/OS association is stronger for 6 years OS and a 6 years follow-up is recommended to assess OS benefit.[7]
Table 1
Phase III trials of adjuvant therapy in stage II/III colon cancer
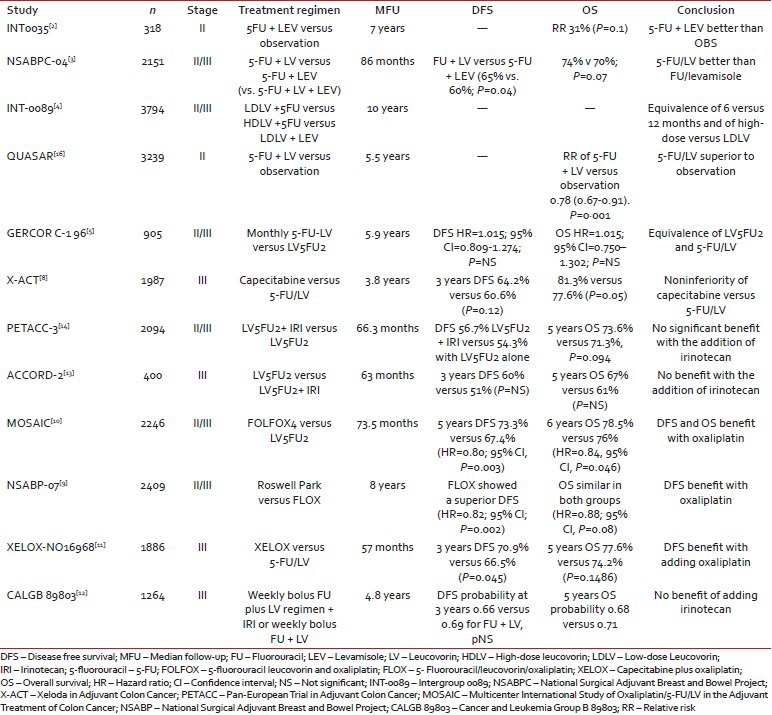
|
In the Multicenter International Study of Oxaliplatin/5FU/LV in the Adjuvant Treatment of Colon Cancer (MOSAIC) phase III trial, 2246 patients with resected stage II/III colon cancer were randomly assigned to receive biweekly infusional LV5FU2 or the same plus oxaliplatin-FOLFOX4 - for 12 cycles (6 months). The 5 years DFS rates were significantly improved with the addition of oxaliplatin, 73.3% and 67.4% in the FOLFOX4 and LV5-FU2 groups respectively (HR = 0.80; 95% confidence interval [CI]: P = 0.003) and the 6 years OS rates were 78.5% and 76% in the FOLFOX4 and LV5FU2 groups, respectively (HR = 0.84, 95% CI: P = 0.046). The contribution of oxaliplatin was confirmed in a phase III NSABP C-07 trial and the XELOXA study,[9,11] firmly establishing the standard of 5-FU + oxaliplatin.
IRINOTECAN IN THE ADJUVANT SETTING
While the role of irinotecan is established in metastatic CRC, its contribution in the adjuvant setting has taken a backseat following the results of three randomized trials failing to show a significant benefit. The Cancer and Leukemia Group B 89803 trial randomly assigned 1264 patients with stage III colon cancer to receive bolus 5-FU/LV alone or with 125 mg/m2 of irinotecan given for 4 weeks with 2 weeks off (irinotecan/fluorouracil/leucovorin regimen). After a median follow-up of 2.6 years, no difference in DFS or OS was observed.[12]
The ACCORD 2 study compared the LV5FU2 regimen with LV5FU2 plus 180 mg/m2 of irinotecan given on day 1 of every cycle for 6 months. DFS was not improved with the LV5FU2/irinotecan combination when compared with infusional 5-FU/LV (51% vs. 60%, respectively).[13] Finally, the Pan-European Trial in Adjuvant Colon Cancer trial (PETACC-3) included over 3000 patients with stage II/III colon cancer who were randomized to receive LV5FU2 with or without irinotecan. Three year OS for stage III disease was 63.3% for patients given LV5FU2/irinotecan versus 60.3% for those in the LV5FU2 arm (HR: 0.89; 95% CI: 0.77-1.11; P = 0.091) and DFS for stage II or III patients was 69.6% versus 66.8% in favor of the irinotecan arm.[14] Given these data, there is currently no established role for irinotecan in the adjuvant setting.
TREATMENT OF STAGE II COLON CANCER
The treatment of node-negative or stage II colon cancer has been long debated with conflicting reports of modest benefit in varied subgroup and meta-analyses reported to date. In a recent analysis of 24,847 patients with stage II colon cancer from the SEER Medicare database, adjuvant 5-FU chemotherapy was not associated with a 5 years OS benefit over observation, even in patients with stage II disease with one or more poor prognostic features (HR: 1.03; 95% CI: 0.94-1.13).[15]
The QUASAR trial[16] stands out as the single randomized trial supporting the role of adjuvant chemotherapy in this setting. In this trial, patients with an “uncertain indication for chemotherapy” were randomized to 5-FU/LV or observation. The majority of enrolled patients were stage II colon cancer. A statistically significant OS benefit for patients with stage II disease treated with 5-FU/LV compared to observation (HR: 0.71; 95% CI: 0.54-0.92; P = 0.01) was reported.
Risk-stratification of stage II disease has emerged as a recommended strategy for adjuvant therapy decision-making. Obstruction, perforation, emergent admission, T4 stage, resection of fewer than 12 lymph nodes, and poor histology are considered high-risk features in stage II and warrant consideration of adjuvant chemotherapy. In the MOSAIC trial, a subset analysis amongst stage II patients showed a DFS of 83.7% and 79.9% in the FOLFOX4 and LV5FU2 arms respectively (HR = 0.84; 95% CI: 0.62 – 1.14). Six years OS in stage III was 72.9% and 68.7% in FOLFOX4 and LV5FU2 respectively (HR = 0.80; 95% CI, P – 0.023). However, there was no OS difference in stage II. The American Society of Clinical Oncology practice guidelines for stage II colon cancer acknowledge that randomized controlled trials have failed to detect a survival benefit for adjuvant chemotherapy in stage II colon cancer. In high-risk stage II, the absolute benefit of 5-FU based therapy is estimated 2-4% and the decision to treat with 5-FU based therapy should be individualized based on disease characteristics, molecular predictive and prognostic factors, co-morbidities, patient preference and risks associated with chemotherapy.[17]
THE IMPORTANCE OF MISMATCH REPAIR AS A PROGNOSTIC BIOMARKER
There are two distinct pathways in CRC pathogenesis: The chromosomal instability (CIN) and microsatellite instability (MSI) which is associated with a CpG island methylator phenotype (CIMP) pathway and deficient mismatch repair (dMMR). More than 70% of CRC arises through the CIN pathway characterized by aneuploidy and loss of heterozygosity and associated with poorer survival.
Defective mismatch repair results from frequent mutations and instability of short tandem repeats resulting in the instability of the genome. Mutations in DNA mismatch repair genes (MLH1, MSH2, MSH6) result in deficient DNA mismatch repair (dMMR) leading to MSI. MMR deficiency can also result from epigenetic silencing of the promoter region of MMR genes by CpG island hypermethylation (CIMP). MSI represents 15% of all sporadic CRCs and 90% of patients with hereditary nonpolyposis colorectal carcinoma. The CIMP pathway is associated with epigenetic inactivation of the MMR genes, most commonly MLH1. dMMR tumors have unique clinic pathological features-younger patients, female preponderance lower stage, proximal location in the right colon, poorly differentiated, mucinous histology and lymphocytic infiltration. Lindor et al. demonstrated that dMMR (tested by IHC) is 97% concordant with MSI-H by polymerase chain reaction (PCR).[21]
In separate reports, Ribic et al. and Sargent et al. demonstrated that 5-FU based chemotherapy is beneficial in stage II/III colon cancer exhibiting MSS or MSI-L but not in MSI-H.[19,20] More recently, Sargent et al. presented a pooled analysis from the ACCENT dataset involving 37,800 patients from 26 randomized colon cancer trials with MMR data on 7803 patients. On a median follow-up of 7 years, in stage II, surgery alone showed a 5 years OS of 90% in d MMR versus 78% in pMMR respectively (P = 0.013). With 5-FU based chemotherapy in stage II, 5 years OS were 88% in d MMR versus 87% in pMMR. Similarly in stage III, surgery alone showed OS of 59% in dMMR versus 54% in proficient MMR (pMMR). In stage III, with 5-FU based therapy-the 5 years OS in dMMR and pMMR were 77% and 71%. These data reaffirm our current understanding that the dMMR is a favorable prognostic feature and in patients with dMMR stage II disease, 5-FU adjuvant chemotherapy is not recommended.[22]
THE ROLE OF ONCOTYPE DX
Oncotype DX is a quantitative multi-gene, real-time PCR assay to measure the gene expression in paraffin-embedded tumor tissues. It is a validated predictor of risk of recurrence in stage II CRC patients following surgery and also a tool for decision-making for the need for adjuvant chemotherapy. Initially developed as an 18-gene panel that included 7 genes for a recurrence score (RS), 6 genes to predict response to 5-FU/LV chemotherapy-treatment score, and 5 reference genes. Subsequent studies revealed that the TS was a not a predictor of response to therapy. Hence, the current oncotype DX is a 12 gene prognostic assay. This assay was validated using the QUASAR trial, where patients were randomized to receive 5-FU/LV or observation after surgical resection. The RS was a strong predictor of recurrence risk (P = 0.004), shorter DFS (P = 0.01) and OS (P = 0.04), with a linear correlation between risk of recurrence and increasing RS. The reported risk of recurrence was 12% at 3 years for the low RS patients, 18% for the intermediate RS patients, and 22% for the high RS patients.[23]
Srivastava et al. in 2014 evaluated the impact of a RS results on physician recommendations regarding adjuvant chemotherapy in T3, mismatch repair-proficient (MMR-P) stage II colon cancer patients. RS was helpful in clinical decision-making associated with treatment recommendation changes for 45% of T3 MMR-P stage II colon cancer patients.[24] Using oncotype DX can lead to a reduction in adjuvant chemotherapy use in this select group of stage II colon cancer patients.
TARGETED THERAPY FOR ADJUVANT TREATMENT-WHERE DO WE STAND?
Likely the biggest disappointment in this decade has been the inability to improve upon the outcomes of patients with resected colon cancer with targeted therapies. Large randomized phase III trials including FU-oxaliplatin chemotherapy with or without bevacizumab (AVANT, NSABP C08)[25,26] and with or without cetuximab (N0147, PETACC8)[27,28] failed to demonstrate improvements in DFS or OS. These trials are summarized in Table 2. Various hypotheses including the potentially limited contribution of the epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) pathways in micrometastatic disease, limitations in our understanding of the epithelial mesenchymal transition, and rebound angiogenesis have been considered. Nonetheless, there is no indication for the use of VEGF- or EGFR-directed targeted therapies in the adjuvant management of colon cancer.
Table 2
Summary of targeted therapy trials in the adjuvant setting
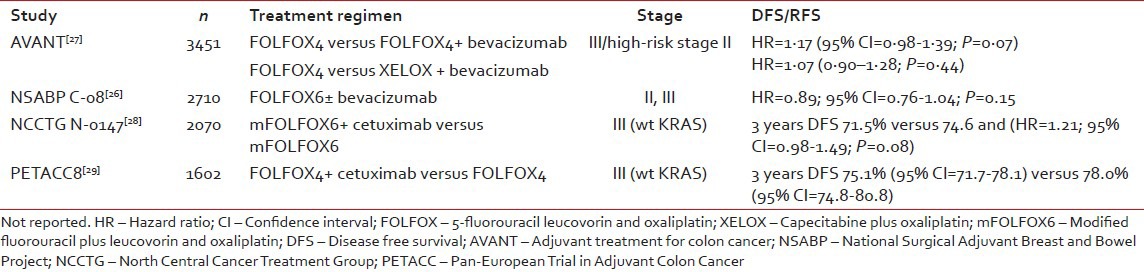
|
REATMENT OF THE ELDERLY
Colorectal cancer is a disease of the elderly with 40% of new cases diagnosed over the age of 75 years. This population is typically under-represented in clinical trials and the age-cut off for “elderly” remains debated. While there has been no evidence to suggest a differential treatment effect in the elderly with 5-FU based chemotherapy[30] there has been conflicting evidence regarding the value of the addition of oxaliplatin in this population.[31] Sanoff et al. in 2012 reported a pooled analysis of patients from 4 databases, which showed an OS difference in patients ≥75 years in stage III CRC with adjuvant chemotherapy. Subset analysis also revealed the benefit of 5% in 3 years OS with oxaliplatin based regimen.[32] There is now an increasing appreciation for the difference between biological age and chronological age, recognizing that geriatric functional assessments can be invaluable in order to determine an individual patient's suitability for adjuvant chemotherapy. Co-morbid factors and their impact on survival should be assessed. We also need to incorporate an estimate the risk of treatment related toxicity and understand patient preference when arriving at a treatment decision. The use of 5-FU based monotherapy is a very reasonable consideration in the geriatric patient population with high-risk resected disease. The recommended adding should be individualized after weighing the risk benefit ratio for a given patient.
DURATION OF ADJUVANT CHEMOTHERAPY
While 6 months of adjuvant chemotherapy remains the current standard, the optimal duration is being actively explored. The International Duration Evaluation of adjuvant chemotherapy (IDEA) Collaboration is an on-going and unique international initiative incorporating patients from six international randomised trials of more than 8500 stage III colon cancer patients. It is designed to assess whether a 3 months course of oxaliplatin-based adjuvant therapy (FOLFOX4/modified FOLFOX6 or XELOX) is equivalent to the current of 6 months treatment for patients with stage III colon cancer, with a primary endpoint of 3 years DFS. As many of the included trials have already completed accrual, the results of this multinational collaborative effort are eagerly awaited.[33]
SUMMARY
Currently, patients with resected stage II disease with high-risk features, and patients with resected stage III disease should be offered adjuvant chemotherapy with 6 months of FOLFOX or XELOX. Patients unsuitable for intensive therapy may be offered 5-FU monotherapy with LV5FU2 or capecitabine. Patients with low-risk stage II disease may be offered the observation or considered for 5-FU monotherapy after a careful discussion regarding risks and uncertain benefits in this indication. It is recommended that MMR testing be routinely performed in all resected stage II colon cancers, as this represents a favorable prognosis and a predictor of poor response to 5-FU chemotherapy. Prognostication by Oncotype DX may further improve our adjuvant decision-making. Presently, there is no indication to support the use of irinotecan, cetuximab or bevacizumab in the adjuvant setting.
While the standard of care for high-risk resected colon cancer has not changed since the addition of oxaliplatin to 5-FU, there has been considerable research in the field exploring opportunities for novel therapies, improved risk-stratification by genomic profiling and an improved understanding of the efficacy of adjuvant therapy in elderly populations. On-going studies of interest include the IDEA collaboration, establishing the duration of adjuvant therapy. Clinical trials in the adjuvant setting should continue to be supported whenever possible.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012. Vol 1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer. Available from http://globocan.iarc.fr.
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352-8.
- Wolmark N, Rockette H, Mamounas E, Jones J, Wieand S, Wickerham DL, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes′ B and C carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol 1999;17:3553-9.
- Haller DG, Catalano PJ, Macdonald JS, O′Rourke MA, Frontiera MS, Jackson DV, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: Final report of Intergroup 0089. J Clin Oncol 2005;23:8671-8.
- André T, Quinaux E, Louvet C, Colin P, Gamelin E, Bouche O, et al. Phase III study comparing a semimonthly with a monthly regimen of fluorouracil and leucovorin as adjuvant treatment for stage II and III colon cancer patients: Final results of GERCOR C96.1. J Clin Oncol 2007;25:3732-8.
- Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much? J Clin Oncol 2004;22:1797-806.
- Sargent D, Sobrero A, Grothey A, O′Connell MJ, Buyse M, Andre T, et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2009;27:872-7.
- Twelves C, Wong A, Nowacki MP, Abt M, Burris H 3 rd , Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696-704.
- Yothers G, O′Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768-74.
- André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16.
- Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465-71.
- Saltz LB, Niedzwiecki D, Hollis D, Goldberg RM, Hantel A, Thomas JP, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J Clin Oncol 2007;25:3456-61.
- Ychou M, Raoul JL, Douillard JY, Gourgou-Bourgade S, Bugat R, Mineur L, et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802). Ann Oncol 2009;20:674-80.
- Van Cutsem E, Labianca R, Bodoky G, Barone C, Aranda E, Nordlinger B, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol 2009;27:3117-25.
- O′Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 2011;29:3381-8.
- Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007;370:2020-9.
- Benson AB 3 rd , Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 2004;22:3408-19.
- Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010;138:2059-72.
- Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219-26.
- Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247-57.
- Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 2002;20:1043-8.
- Sargent DJ, Shi Q, Yothers G, Tejpar S, Bertagnolli M, Thibodeau S, et al. Prognostic impact of deficient mismatch repair in 7,803 stage II/III colon cancer patients: A pooled individual pt data analysis of 17 adjuvant trials in the ACCENT database. J Clin Oncol 2014;32:52.
- Gray RG, Quirke P, Handley K, Lopatin M, Magill L, Baehner FL, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol 2011;29:4611-9.
- Srivastava G, Renfro LA, Behrens RJ, Lopatin M, Chao C, Soori GS, et al. Prospective multicenter study of the impact of oncotype DX colon cancer assay results on treatment recommendations in stage II colon cancer patients. Oncologist 2014;19:492-7.
- Salazar R, Roepman P, Capella G, Moreno V, Simon I, Dreezen C, et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J Clin Oncol 2011;29:17-24.
- Allegra CJ, Yothers G, O′Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. J Clin Oncol 2011;29:11-6.
- de Gramont A, Van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): A phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225-33.
- Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: A randomized trial. JAMA 2012;307:1383-93.
- Taieb J, Tabernero J, Mini E, Subtil F, Folprecht G, Van Laethem J, et al. Adjuvant FOLFOX4 with or without cetuximab in patietns with resected stage III colon cancer: (PETACC-8): an open-label, randomised phase 3 trial.The Lancet Oncology 2014;15:862-73.
- Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091-7.
- McCleary NJ, Meyerhardt JA, Green E, Yothers G, de Gramont A, Van Cutsem E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: Findings from the ACCENT database. J Clin Oncol 2013;31:2600-6.
- Sanoff HK, Carpenter WR, Stürmer T, Goldberg RM, Martin CF, Fine JP, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol 2012;30:2624-34.
- André T, Iveson T, Labianca R, Meyerhardt JA, Souglakos I, Yoshino T, et al. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) collaboration: Prospective combined analysis of phase III trials investigating duration of adjuvant therapy with the FOLFOX (FOLFOX4 or Modified FOLFOX6) or XELOX (3 versus 6 months) regimen for patients with stage III colon cancer: Trial design and current status. Curr Colorectal Cancer Rep 2013;9:261-9.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012. Vol 1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer. Available from http://globocan.iarc.fr.
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352-8.
- Wolmark N, Rockette H, Mamounas E, Jones J, Wieand S, Wickerham DL, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes′ B and C carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol 1999;17:3553-9.
- Haller DG, Catalano PJ, Macdonald JS, O′Rourke MA, Frontiera MS, Jackson DV, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: Final report of Intergroup 0089. J Clin Oncol 2005;23:8671-8.
- André T, Quinaux E, Louvet C, Colin P, Gamelin E, Bouche O, et al. Phase III study comparing a semimonthly with a monthly regimen of fluorouracil and leucovorin as adjuvant treatment for stage II and III colon cancer patients: Final results of GERCOR C96.1. J Clin Oncol 2007;25:3732-8.
- Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much? J Clin Oncol 2004;22:1797-806.
- Sargent D, Sobrero A, Grothey A, O′Connell MJ, Buyse M, Andre T, et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2009;27:872-7.
- Twelves C, Wong A, Nowacki MP, Abt M, Burris H 3 rd , Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696-704.
- Yothers G, O′Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768-74.
- André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16.
- Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465-71.
- Saltz LB, Niedzwiecki D, Hollis D, Goldberg RM, Hantel A, Thomas JP, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J Clin Oncol 2007;25:3456-61.
- Ychou M, Raoul JL, Douillard JY, Gourgou-Bourgade S, Bugat R, Mineur L, et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802). Ann Oncol 2009;20:674-80.
- Van Cutsem E, Labianca R, Bodoky G, Barone C, Aranda E, Nordlinger B, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol 2009;27:3117-25.
- O′Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 2011;29:3381-8.
- Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007;370:2020-9.
- Benson AB 3 rd , Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 2004;22:3408-19.
- Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010;138:2059-72.
- Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219-26.
- Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247-57.
- Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 2002;20:1043-8.
- Sargent DJ, Shi Q, Yothers G, Tejpar S, Bertagnolli M, Thibodeau S, et al. Prognostic impact of deficient mismatch repair in 7,803 stage II/III colon cancer patients: A pooled individual pt data analysis of 17 adjuvant trials in the ACCENT database. J Clin Oncol 2014;32:52.
- Gray RG, Quirke P, Handley K, Lopatin M, Magill L, Baehner FL, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol 2011;29:4611-9.
- Srivastava G, Renfro LA, Behrens RJ, Lopatin M, Chao C, Soori GS, et al. Prospective multicenter study of the impact of oncotype DX colon cancer assay results on treatment recommendations in stage II colon cancer patients. Oncologist 2014;19:492-7.
- Salazar R, Roepman P, Capella G, Moreno V, Simon I, Dreezen C, et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J Clin Oncol 2011;29:17-24.
- Allegra CJ, Yothers G, O′Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. J Clin Oncol 2011;29:11-6.
- de Gramont A, Van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): A phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225-33.
- Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: A randomized trial. JAMA 2012;307:1383-93.
- Taieb J, Tabernero J, Mini E, Subtil F, Folprecht G, Van Laethem J, et al. Adjuvant FOLFOX4 with or without cetuximab in patietns with resected stage III colon cancer: (PETACC-8): an open-label, randomised phase 3 trial.The Lancet Oncology 2014;15:862-73.
- Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091-7.
- McCleary NJ, Meyerhardt JA, Green E, Yothers G, de Gramont A, Van Cutsem E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: Findings from the ACCENT database. J Clin Oncol 2013;31:2600-6.
- Sanoff HK, Carpenter WR, Stürmer T, Goldberg RM, Martin CF, Fine JP, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol 2012;30:2624-34.
- André T, Iveson T, Labianca R, Meyerhardt JA, Souglakos I, Yoshino T, et al. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) collaboration: Prospective combined analysis of phase III trials investigating duration of adjuvant therapy with the FOLFOX (FOLFOX4 or Modified FOLFOX6) or XELOX (3 versus 6 months) regimen for patients with stage III colon cancer: Trial design and current status. Curr Colorectal Cancer Rep 2013;9:261-9.


 PDF
PDF  Views
Views  Share
Share

