Adjuvant brachytherapy for Stage IB Grade 2 endometrial carcinoma: Multivariate analysis of a single institution experience
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(02): 112-115
DOI: DOI: 10.4103/0971-5851.180148
Abstract
Objective: The aim was to investigate the value of postoperative brachytherapy for patients with Stage IB, Grade 2 endometrial carcinoma. Patients and Methods: Forty-six patients with Stage IB, Grade 2 endometrial carcinoma, were treated with simple hysterectomy and bilateral oophorectomy in our institution. The mean age was 63 (range, 42-81). Surgical staging, defined as peritoneal washing and pelvic lymph node sampling was performed in 73% of patients. Twenty-two patients (47%) received a postoperative intravaginal brachytherapy (IVRT), and 24 patients (53%) were followed-up without additional treatment. Results: The median follow-up was 60 months. The 5-year overall survival for irradiated and nonirradiated patients, was 83.5 and 94.7%, respectively. Four patients (8.7%) developed relapse, two in the group of postoperative IVRT and 2 in the follow-up only group. Multivariate analysis demonstrated a borderline association (P = 0.06) between lower uterine segment involvement and poor pelvic-vaginal control. The presence of GOG #99 high-risk features did not affect the pelvic control rate. Conclusion: According to our experience and previously published data, most patients with FIGO Stage IB, Grade 2 endometrial carcinoma may be cured with surgery alone.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Objective:
The aim was to investigate the value of postoperative brachytherapy for patients with Stage IB, Grade 2 endometrial carcinoma.
Patients and Methods:
Forty-six patients with Stage IB, Grade 2 endometrial carcinoma, were treated with simple hysterectomy and bilateral oophorectomy in our institution. The mean age was 63 (range, 42-81). Surgical staging, defined as peritoneal washing and pelvic lymph node sampling was performed in 73% of patients. Twenty-two patients (47%) received a postoperative intravaginal brachytherapy (IVRT), and 24 patients (53%) were followed-up without additional treatment.
Results:
The median follow-up was 60 months. The 5-year overall survival for irradiated and nonirradiated patients, was 83.5 and 94.7%, respectively. Four patients (8.7%) developed relapse, two in the group of postoperative IVRT and 2 in the follow-up only group. Multivariate analysis demonstrated a borderline association (P = 0.06) between lower uterine segment involvement and poor pelvic-vaginal control. The presence of GOG #99 high-risk features did not affect the pelvic control rate.
Conclusion:
According to our experience and previously published data, most patients with FIGO Stage IB, Grade 2 endometrial carcinoma may be cured with surgery alone.
INTRODUCTION
Endometrial cancer is the fourth most common female malignancy and is the most common gynecological cancer in the United States. In 2006, an estimated 41,200 new cases occurred, accounting for approximately 6% of diagnosed female cancers. Endometrial cancer was estimated to have resulted in 3% of female cancer deaths in 2006.[1]
However, the optimal adjuvant treatment for early-stage endometrial carcinoma remains undefined despite published results from randomized trials.[2] Recent publications have revealed 5-year overall survival (OS) rates of 80% to 90% for women with stage I disease who underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH-BSO) and adjuvant radiation therapy (XRT).[3] Controversy exists over how XRT should be administered in the patients with stage I endometrial cancer. Numerous reports have focused on early-stage patients treated with postoperative XRT. These reports vary greatly. In some intravaginal brachytherapy (IVRT) is used while, in others, all patients undergo pelvic irradiation therapy (PRT). Despite such differences, pelvic/vaginal control rates exceed 95% in the great majority of reports.
Patients with Stage IB Grade 2 endometrial cancer are considered to have low-risk disease. Although some investigators recommend no postoperative treatment, others favor adjuvant radiotherapy, either PRT or IVRT.
The purpose of this study was to evaluate the impact of adjuvant IVRT on local control and survival for patients with Stage IB Grade 2 endometrial cancer, after TAH-BSO.
PATIENTS AND METHODS
The patient population consisted of 46 patients with FIGO Stage IB Grade 2 (moderately differentiated) endometrial cancer, who were treated at the Soroka Medical Center between February 1990 and October 2001. All patients had endometrial adenocarcinoma FIGO Stage IB Grade 2. Patients with any component of papillary serous or clear cell histology were excluded from our analysis.
The mean age was 63 (range, 42-81), [Table 1].
Table 1
Patient characteristics

All patients underwent TAH-BSO. Pelvic washings were obtained in 40 patients (87%). Surgical staging, defined as pelvic washings and pelvic lymph node sampling, was performed in 33 patients (72%). The indications for surgical staging were generally determined by the depth of invasion and the tumor grade.
Twenty-two (47%) patients received postoperative low-dose rate intravaginal radiotherapy (LDR-IVRT), and 24 (53%) had received no further treatment.
The total dose of postoperative LDR-IVRT was 8 Gy, delivered in two fractions over a 2 week interval. The target volume treated was one-half to two-thirds of the length of the vagina. The treatment was delivered using a cylinder loaded by 137Cs source. The typical dose per fraction was 4 Gy. The dose was prescribed to a depth of 0.25 cm from the vaginal surface. The median follow-up time was 60 months.
Survival rates were calculated using the Kaplan–Meier method. Independent prognostic factors for survival were analyzed using the Cox proportional hazards model. The probability of relapse was estimated using logistic regression model.
RESULTS
After a median follow-up of 5-year (range, 0.5-15.1 years), 38 (82.6%) of the 46 patients were alive.
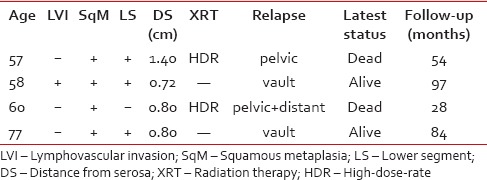
Four patients (8.7%) developed relapse, two in the group of postoperative IVRT and two in the follow-up only group. Their clinical, pathological, and treatment characteristics are shown in Table 2. The site of relapse was the vaginal vault in 2 patients (4.4%), the pelvis in one patient (2.2%) and combined pelvic/distant in 1 patient (2.2%). Two isolated vaginal vault recurrences were successfully controlled by PRT alone and both women are alive with stable disease. One patient with pelvic/distant disease received PRT and systemic chemotherapy, but eventually died with progressive disease.
Table 2
Characteristics of relapsed patients
The estimated 5-year survival for the irradiated and nonirradiated groups was 83.52 and 94.74%, respectively [Figure 1]. Log-rank analysis of survival showed no significant difference between the irradiated (mean: 10.6 years; 95% CI, 8.4/12.7) and nonirradiated groups of the patients (mean: 12.8 years; 95% CI, 10.7/14.9; P = 0.45).
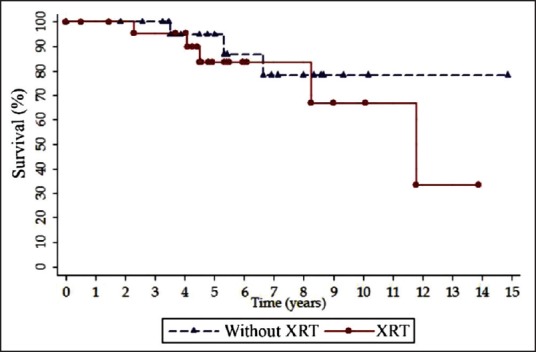
| Fig. 1 Kaplan–Meier curves for overall survival by radiation therapy
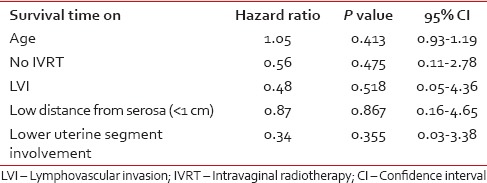
The GOG #99 studies defined a number of risk factors for early-stage endometrial cancer such as age, lymph – vascular space invasion (LVI), depth of myometrial invasion and Grade 2 or 3 tumors. The depth of myometrial invasion and the tumor grade were predetermined in our study. LVI, age, lower uterine segment involvement and distance from serosa were analyzed for relationship with survival time using Cox proportional hazards model. Hazard ratio estimates for each prognostic factor are shown in Table 3. No, statistically significant effect on survival was detected. Postoperative IVRT was also shown to have no prognostic significance in terms of local control and survival.
Table 3
Relationship of adverse prognostic factor with survival
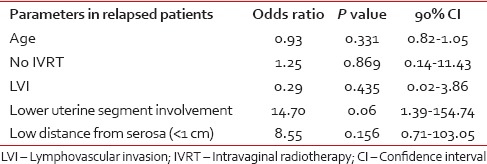
Logistic regression model showed that postoperative IVRT or patient's age, LVI, lower uterine segment involvement, distance from serosa did not significantly affect pelvic relapse rate. Multivariate analysis demonstrated a borderline association between lower uterine segment involvement and poor pelvic-vaginal control (or, 14.7; 90% CI, 1.39/154.73; P = 0.06) [Table 4].
Table 4
Regression analysis of association between patient parameters and risk of recurrence
DISCUSSION
The role of adjuvant PRT in early-stage endometrial cancer was evaluated in two large randomized trials. To determine which groups of patients are at high-risk of recurrence, GOG #99 separated the patient population into high-risk versus low-risk, according to advanced age, Grade 2 or 3 tumors, lymph-vascular invasion and outer one-third myometrial invasion.[4] Nearly 60% of patients in the GOG #99 trial were Stage IB, and approximately 80% had Grade 1 or 2 disease. The Postoperative Radiation Therapy in Endometrial Cancer trial included 715 patients with stage 1C, Grade 1 or 2, and Stage IB, Grade 2 or 3 endometrial cancer.[5] A total of 221 (30%) patients with stage 1B Grade 2 were evaluated in this trial. These two randomized trials concluded that PRT for stage I endometrial cancer leads to improved loco-regional control but had no impact on OS.
Many series have reported an excellent outcome in Stage IB Grade 1 or 2 disease without adjuvant XRT.[6,7,8] In surgically staged patients, Straughn, et al.[8] reported a 3.7% overall failure rate in 296 patients, with 64% of the recurrences being at the vaginal vault. Other series have reported similar outcomes in patients who did not undergo surgical staging. The Mayo clinic reported their experience in 261 patients with Grade 1 or 2 and Stage IB disease treated with surgery alone. There was only a 2% isolated local recurrence rate without adjuvant XTR.[7] Given the low-risk of pelvic failure, some investigators recommended IVRT as adjuvant treatment for this group of patients.[9,10] The largest series was reported by Alektiar et al.[11] In 233 patients without surgical staging there was an overall 4% relapse rate, only 2% failed in the pelvis.
Taken together, these data do not support the use of postoperative pelvic XRT for the low-risk patients. Observation alone is appropriate for Stage IB Grade 1 disease and observation or IVRT is a reasonable treatment option for Stage IB, Grade 2 disease.
Recently, a large population analysis of adjuvant XRT in early-stage endometrial cancer was published.[3] This study included 21 249 women with Stage IA-IC endometrial cancer. A total of 4080 (19.2%) women received postoperative XRT. Of these patients, 2551 (62.5%) had pelvic XRT, 732 (17.9%) had IVRT, and 1078 (26.4%) received combination of EBRT with IVBT. Of 4316 patients with Stage IB Grade 2, 751 (17.4%) received adjuvant XRT. The authors concluded that adjuvant XRT has no impact on OS for this group of patients, except the patients older than age 75 years old.
The results of the present study suggest that postoperative IVRT has no significant impact on local control and survival of patients with Stage IB Grade 2 endometrial cancer.
Based on our experience and previous publication the surgically staged patients with Stage IB Grade 2 endometrial cancer without additional risk factors may be treated with TAH-BSO without postoperative XRT.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 Kaplan–Meier curves for overall survival by radiation therapy


 PDF
PDF  Views
Views  Share
Share

