Acute Leukemia Presenting with Musculoskeletal Manifestations: A Case Series
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2020; 41(01): 29-33
DOI: DOI: 10.4103/ijmpo.ijmpo_113_19
Abstract
Introduction:?Leukemia is the most common childhood malignancy accounting for 30%?40% of cases. Acute lymphoblastic leukemia is the most common leukemia in children with peak incidence in 2?6 years of age. The present study aims to assess the incidence of acute leukemia in patients presenting with musculoskeletal manifestations.?Materials and Methods:?This is a retrospective study conducted in a tertiary center from January 2014 to December 2018. A total of 63 children presented with musculoskeletal manifestations and underwent bone marrow examination. Based on final marrow diagnosis, the study group was divided into leukemic and nonleukemic groups.?Results:?Fever was the most common presenting complaint and was present in all the patients of both the groups. The occurrence of hepatosplenomegaly was comparatively higher in the leukemic group than in patients with juvenile idiopathic arthritis (JIA). The predominant type of arthritis was oligoarticular (68.15%) in the leukemic group and polyarticular (77.27%) in the nonleukemic group. Rheumatoid rash was noted in 20% of JIA patients, and none of the patients in the leukemic group had rash. The percentages of anemia, leukopenia, and thrombocytopenia were statistically higher in leukemia patients than in JIA patients.?Conclusion:?Bone marrow studies are a prerequisite in diagnosing leukemias. However, based on the presence of few atypical clinical and laboratory features, leukemia can be excluded in JIA patients.
 ?
?Publication History
Received: 07 May 2019
Accepted: 12 July 2019
Publication Date:
23 May 2021 (online)
? 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:?Leukemia is the most common childhood malignancy accounting for 30%?40% of cases. Acute lymphoblastic leukemia is the most common leukemia in children with peak incidence in 2?6 years of age. The present study aims to assess the incidence of acute leukemia in patients presenting with musculoskeletal manifestations.?Materials and Methods:?This is a retrospective study conducted in a tertiary center from January 2014 to December 2018. A total of 63 children presented with musculoskeletal manifestations and underwent bone marrow examination. Based on final marrow diagnosis, the study group was divided into leukemic and nonleukemic groups.?Results:?Fever was the most common presenting complaint and was present in all the patients of both the groups. The occurrence of hepatosplenomegaly was comparatively higher in the leukemic group than in patients with juvenile idiopathic arthritis (JIA). The predominant type of arthritis was oligoarticular (68.15%) in the leukemic group and polyarticular (77.27%) in the nonleukemic group. Rheumatoid rash was noted in 20% of JIA patients, and none of the patients in the leukemic group had rash. The percentages of anemia, leukopenia, and thrombocytopenia were statistically higher in leukemia patients than in JIA patients.?Conclusion:?Bone marrow studies are a prerequisite in diagnosing leukemias. However, based on the presence of few atypical clinical and laboratory features, leukemia can be excluded in JIA patients.
Keywords
Atypical presentation - childhood leukemia - juvenile rheumatoid arthritisIntroduction
Leukemia is the most common childhood malignancy accounting for 30%?40% of cases.[1] Acute lymphoblastic leukemia (ALL) is the most common leukemia in children with peak incidence in 2?6 years of age.[2] Leukemias characteristically present with fever, bleeding manifestations, organomegaly, and deranged hematological profile. Sometimes, leukemia can show heterogeneous clinical course and present with musculoskeletal manifestations in the form of arthralgia or overt arthritis with near-normal hematological profile. Diagnosis of acute leukemia in such cases may be delayed as these may be initially misdiagnosed as juvenile idiopathic arthritis (JIA). With this background, the present study was carried out to assess the incidence of acute leukemia in patients presenting with musculoskeletal manifestations and to assess the clinical and laboratory findings that help in differentiating these two groups.
Materials and Methods
The present study was a retrospective study conducted at a tertiary center from January 2014 to December 2018. A total of 63 children presented with musculoskeletal manifestations and underwent bone marrow examination. We enrolled children below the age of 16 years in this study. Prior informed consent was obtained from the parents/guardian to perform bone marrow aspiration and biopsy studies as well as to use the information obtained for future research purposes. Based on final marrow diagnosis, the study group was divided into leukemic and nonleukemic groups. Clinical records of both the groups were reviewed, and the following data were extracted and statistically compared.
Clinical data
Age, sex, fever (axillary temperature ?37.8?C), lymph node enlargement, hepatomegaly, splenomegaly, type of arthritis (arthritis involving ?4 joints is oligoarthritis and ?5 is polyarthritis), and rheumatoid rash.
Laboratory data
Hemoglobin (Hb), total leukocyte count (TLC), platelet count, erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH) levels, number of blasts in peripheral blood, and bone marrow studies.
Definitions of quantitative hematological parameters
Anemia: Hb <10>12 ? 109/L; neutropenia: absolute neutrophil count (ANC) <1>400 ? 109/L; upper limit for serum LDH: 500 IU/L; high ESR: >20 mm 1st?h; and acute leukemia: >20% blasts in peripheral blood or bone marrow.
Statistical analysis
Data analysis was done, and the results were compared between the two groups. Using Fisher?s exact test with two-sided tests and 5% level of significance, differences in frequencies were tested for statistical significance.
Results
The total number of patients in the study group was 63. Clinical and laboratory data of all the cases were analyzed, and the study group was divided into leukemic and nonleukemic groups based on bone marrow diagnosis. Of these, 30.15% (n?= 19) of 63 cases were diagnosed with leukemia and the rest of the cases accounting to 69.85% (n?= 44) fell into the nonleukemic group who were diagnosed and treated as JIA. Among patients diagnosed with leukemia, ALL was the most common diagnosis seen in 89.48% (n?= 17) of cases. Among the nonleukemic group, 59.09% (n?= 26) of cases showed normal marrow on bone marrow examination, as depicted in [Table 1].
Table 1Distribution of cases in the study group into leukemic and nonleukemic groups
|
Sub group |
No. of cases (%) |
|---|---|
|
ALL ? Acute lymphoblastic leukemia; AML ? Acute myeloid leukemia |
|
|
Leukemic group |
19 (30.15) |
|
ALL |
17 (89.48) |
|
AML |
2 (10.52) |
|
Nonleukemic group |
44 (69.85) |
|
Normal marrow |
26 (59.09) |
|
Reactive marrow |
18 (40.91) |
|
Total number of cases |
63 (100) |
Demographic data
The median age at diagnosis was significantly higher in the leukemic group compared to the nonleukemic group (9 [4?16] vs. 6.1 [2?15] years, respectively) with?P?= 0.0118. No statistical difference was observed in gender for both the groups, with M: F of 1.5?1:1.
Clinical data
The occurrence of clinical manifestations in both the groups was assessed and compared, as shown in [Table 2]. Fever was the most common presenting complaint and was present in all the patients of both the groups, and there were no differences observed in the frequencies of arthralgia in both the groups. The occurrence of hepatomegaly and splenomegaly was comparatively higher in the leukemic group than in patients with JIA (42.1% vs. 4.54%,?P?= 0.008, and 47.36% vs. 6.81%,?P?= 0.0006, respectively). On comparison of musculoskeletal manifestations, the predominant type of arthritis was oligoarticular (68.15%) in the leukemic group and polyarticular (77.27%) in the nonleukemic group. The occurrence of lymphadenopathy was almost equal in both the groups with an insignificant?P?value (P?= 0.9878). Rheumatoid rash was noted in 20% of JIA patients, and none of the patients in the leukemic group had rash.
Laboratory data
The laboratory parameters between the two study groups were assessed, and the median hemoglobin, white blood cell count, ANC, and platelet count were significantly lower in leukemia patients than in JIA patients (6.7 g/dl vs. 10.2 g/dl, 4400/mm3?vs. 15,600/mm3, 0.7 ? 103/mm3?vs. 6.9 ? 103/mm3, and 56,000/mm3?vs. 3.8 lakhs/mm3, respectively).
The percentages of anemia, leukopenia, and thrombocytopenia were statistically higher in leukemia patients than in JIA patients (36.84% vs. 9.09%,?P?0.0313; 47.3% vs. 13.63%,?P?0.0081; and 57.89% vs. 9.09%,?P?< 0 href="https://www.thieme-connect.com/products/ejournals/html/10.4103/ijmpo.ijmpo_113_19#FI_1" xss=removed>Figure 1]. All cases with leukemia showed thrombocytopenia, whereas thrombocytosis was seen only in nonleukemic patients, as shown in [Table 3]. Increased LDH levels were seen in 63% of leukemia patients, and high ESR was noted in 75% of JIA patients.
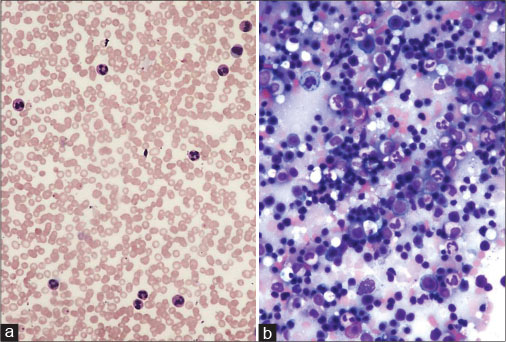
|?Fig. 1: (a) Juvenile idiopathic arthritis ? peripheral smear shows neutrophilic leukocytosis ? Giemsa, ?100. (b) Reactive marrow and absence of blasts ? Giemsa, ?100
Of 19 cases of leukemia, 17 were diagnosed as ALL and 2 as acute myeloid leukemia. Seven patients (36.8%) presented with peripheral pancytopenia with no blasts in peripheral blood [Figure 2] and 12 patients (63.2%) presented with bi/monocytopenia and few blasts in the peripheral blood. Immunophenotyping revealed that the frequencies of the presence of musculoskeletal manifestations were similar in both B- Acute lymphoblastic leukemia and T-Acute lymphoblastic leukemia.
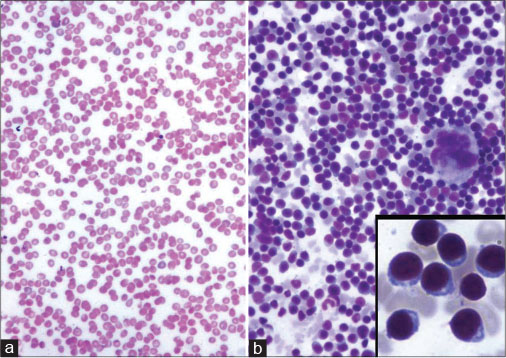
|?Fig. 20: Acute leukemia (a) Peripheral pancytopenia ? Giemsa, ?100. (b) Hypercellular marrow with prominence of blasts ? Giemsa, ?100 (inset shows blasts ? Giemsa, ?400)
Discussion
Leukemia is the most common pediatric cancer in many countries such as India, Brazil, Canada, and the United States.[1] [2] [3] More than 80% of all childhood cancer cases occur in low- and middle-income countries.[4] In India, the highest incidence was seen in Delhi at age-adjusted incidence rates of 101.3/million in boys and 62.3/million in girls.[5] The clinical picture of acute leukemia in children constitutes varied manifestations unlike adults. The disease can present with musculoskeletal findings mimicking JIA, rheumatic fever, juvenile systemic lupus erythematosus, and vasculitis.[6] [7] [8] [9] [10] [11] [12] [13]
The present study was done to evaluate and investigate the significance of musculoskeletal features as a presenting manifestation of leukemia in children who were misdiagnosed as JIA. JIA is the most common chronic rheumatologic disease in children. Characteristic features of JIA include arthritis for at least 6 weeks, morning stiffness, joint pain or abnormal joint use, spiking fevers, and evanescent rash on the trunk and extremities.[14] [15]
Arthritis or arthritis-like symptoms may be seen in other conditions, and leukemia is the single most important differential diagnosis mistaken for JIA. Children with leukemia can present with various osteoarticular signals and symptoms, including arthralgia, arthritis, and limb pain, which are typically severe and may awaken the child from sleep. This is due to the expansion of lymphoblasts in bone metaphyses.[15] [16]
The subtypes of JIA include systemic-onset JIA (SoJIA), oligoarticular JIA, polyarticular JIA, psoriatic arthritis, enthesitis-related arthritis, and undifferentiated arthritis. SoJIA is an infrequent subtype of JIA and has prominent extra-articular manifestations and a significant elevation in acute-phase reactants.[17] At disease onset, patients with SoJIA may have only systemic manifestations without arthritis, thereby causing diagnostic delay. The possibility of leukemia should be always considered in the differential diagnosis of SoJIA presenting with following atypical, clinical, and laboratory features.
Atypical features of systemic-onset juvenile idiopathic arthritis favoring leukemia
Clinical features
Pain disproportionate to severity of arthritis and predominantly nocturnal, nonarticular bony pain requiring opiate analgesia, sequential improvement with steroids initially with subsequent rapid deterioration, and asymmetrical pauciarticular arthritis.[18]
Laboratory features
Severe anemia, leukopenia, neutropenia with relative lymphocytosis, thrombocytopenia, elevated serum LDH, and acute-phase response disproportionate to severity of arthritis.
In our study, the presence of fever, hepatosplenomegaly, limb pain, and weight loss was significantly noted in 31.5% of patients with leukemia than in JIA patients. These findings significantly correlated with Tamashiro?et al. and Brix?et al. studies.[18] [19] [20] [21]
We also found higher levels of anemia, leukopenia, neutropenia, and thrombocytopenia, in patients with leukemia which was similar to the findings in other studies. Thrombocytopenia with neutropenia and lymphocytosis is rare in children with JIA, and their presence suggests the possibility of leukemia. Tamashiro?et al. found in their study that limb pain and thrombocytopenia are two independent variables associated with leukemia in 91% of their patients.[18] LDH is a marker of increased cell turnover and increased LDH was seen in 63.15% of cases of leukemia which correlates with a study by Brix?et al. and Wallendal?et al., who found that serum LDH levels were significantly higher in cancer patients, including leukemia.[22] A history of nighttime pain was seen in 72.27% of our cases diagnosed with leukemia. The three most important predictive factors for a pediatric leukemia diagnosis as suggested by Jones?et al. are leukopenia, thrombocytopenia, and a history of nighttime pain.[23] In the present study, among the nonleukemic group, fever and rheumatoid rash were predominantly observed in JIA patients. Laboratory data revealed that leukocytosis and thrombocytosis were frequently seen in patients with JIA that could distinguish them from leukemia patients. In the leukemic group, 63.2% of cases showed blasts in periphery. Brix?et al. showed that 70% of the children with ALL with joint involvement had blasts in peripheral blood at admission equivalent to the children with ALL without joint involvement.[19] This discrepancy could be due to the diagnosis of the disease at different stages. A change in methodology over time such as usage of flow cytometry, which is more sensitive to blasts than morphology, also explains the discrepancy.
Our cases were initially diagnosed as JIA based on clinical findings and started on steroids. A study by Barbosa et al. showed that only 13% had clinical evidence of arthritis, at the onset of leukemia, of which 8% were misdiagnosed with rheumatic fever or JIA before referral, and some of these patients had already received steroids, delaying the start of appropriate treatment.[6] Treatment with steroids causes a left shift in the marrow, thereby diagnosis of leukemia was delayed by 1?8 months. Majority (76.47%) of the leukemic group were not showing blasts in the peripheral blood and were diagnosed only after bone marrow studies. The importance of investigating leukemia in children and adolescents who present with musculoskeletal manifestations, especially arthritis and thrombocytopenia, is emphasized in this study.
Conclusion
Bone marrow studies are a prerequisite in diagnosing leukemia in cases with musculoskeletal symptoms and absence of blasts in peripheral blood. Leukemia must be excluded first, as treatment with steroids temporarily reduces the cell counts and thereby masks the symptoms. This further delays a malignancy diagnosis and reduces the subsequent response to chemotherapy. In this study, a few atypical, clinical, and laboratory parameters were demonstrated that differentiate acute leukemia from JIA at disease onset, thereby reducing diagnostic delay.
Conflict of Interest
There are no conflicts of interest.
References
- Linabery AM, Ross JA.?Trends in childhood cancer incidence in the U.S. (1992-2004). Cancer 2008; 112: 416-32
- de Camargo B, de Oliveira Santos M, Rebelo MS, de Souza Reis R, Ferman S, Noronha CP. et al.?Cancer incidence among children and adolescents in Brazil:First report of 14 population-based cancer registries. Int J Cancer 2010; 126: 715-20
- Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK. et al.?Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 2008; 58: 15-25
- Barr R, Riberio R, Agarwal B, Masera G, Hesseling P, Magrath I.?Pediatric oncology in countries with limited resources. In: Pizzo PA, Poplack DG. editors Principles and Practice of Pediatric Oncology. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2006: 1605-17
- Bashar A.?Incidence and pattern of childhood cancers in India: Findings from population-based cancer registries. Indian J Cancer 2016; 53: 511-2
- Barbosa CM, Nakamura C, Terreri MT, Lee ML, Petrilli AS, Hil?rio MO.?Musculoskeletal manifestations as the onset of acute leukemias in childhood. J Pediatr (Rio J) 2002; 78: 481-4
- Robazzi TC, Barreto JH, Silva LR, Santiago MB, Mendon?a N.?Osteoarticular manifestations as initial presentation of acute leukemias in children and adolescents in Bahia, Brazil. J Pediatr Hematol Oncol 2007; 29: 622-6
- Campos LM, Goldstein S, Santiago RA, Jesus AA, Cristofani LM, Odone Filho V. et al.?Musculoskeletal involvement as a first manifestation of neoplasm disease. Rev Assoc Med Bras (1992) 2008; 54: 132-8
- Schaller J.?Arthritis as a presenting manifestation of malignancy in children. J Pediatr 1972; 81: 793-7
- Saulsbury FT, Sabio H.?Acute leukemia presenting as arthritis in children. Clin Pediatr (Phila) 1985; 24: 625-8
- Goncalves M, Terreri MT, Barbosa CM, Len CA, Lee L, Hilario MO.?Diagnosis of malignancies in children with musculoskeletal complaints. Sao Paulo Med J 2005; 123: 21-3
- Murray MJ, Tang T, Ryder C, Mabin D, Nicholson JC.?Childhood leukaemia masquerading as juvenile idiopathic arthritis. BMJ 2004; 329: 959-61
- Tafaghodi F, Aghighi Y, Rokni Yazdi H, Shakiba M, Adibi A.?Predictive plain X-ray findings in distinguishing early stage acute lymphoblastic leukemia from juvenile idiopathic arthritis. Clin Rheumatol 2009; 28: 1253-8
- Marwaha RK, Kulkarni KP, Bansal D, Trehan A.?Acute lymphoblastic leukemia masquerading as juvenile rheumatoid arthritis: Diagnostic pitfall and association with survival. Ann Hematol 2010; 89: 249-54
- Lovell DJ.?Juvenile idiopathic arthritis: Clinical features. In: Kippel JH, Stone JH, Crofford LJ, White PH. editors Primer on the Rheumatic Diseases. 13th ed. New York: Springer Science; 2008
- Simard JF, Neovius M, Hagelberg S, Askling J.?Juvenile idiopathic arthritis and risk of cancer: A nationwide cohort study. Arthritis Rheum 2010; 62: 3776-82
- Yanagimachi M, Miyamae T, Naruto T, Hara T, Kikuchi M, Hara R. et al.?Association of HLA-A*02:06 and HLA-DRB1*04:05 with clinical subtypes of juvenile idiopathic arthritis. J Hum Genet 2011; 56: 196-9
- Tamashiro MS, Aikawa NE, Campos LM, Cristofani LM, Odone-Filho V, Silva CA.?Discrimination of acute lymphoblastic leukemia from systemic-onset juvenile idiopathic arthritis at disease onset. Clinics (Sao Paulo) 2011; 66: 1665-9
- Brix N, Rosth?j S, Herlin T, Hasle H.?Arthritis as presenting manifestation of acute lymphoblastic leukaemia in children. Arch Dis Child 2015; 100: 821-5
- Mulder H, Herregods N, Mondelaers V, Benoit Y, De Moerloose B.?Musculoskeletal manifestations in children with acute lymphoblastic leukaemia. Belg J Hematol 2012; 3: 3-11
- zali S, Prashanth GP, Amarkhed P.?Migratory polyarthritis in aleukemic lymphoblastic leukemia: An undesignated paraneoplastic syndrome. J Sci Soc 2013; 40: 44-6
- ?Wallendal M, Stork L, Hollister JR.?The discriminating value of serum lactate dehydrogenase levels in children with malignant neoplasms presenting as joint pain. Arch Pediatr Adolesc Med 1996; 150: 70-3
- ?Jones OY, Spencer CH, Bowyer SL, Dent PB, Gottlieb BS, Rabinovich CE.?A multicenter case-control study on predictive factors distinguishing childhood leukemia from juvenile rheumatoid arthritis. Pediatrics 2006; 117: e840-4
Address for correspondence
Publication History
Received: 07 May 2019
Accepted: 12 July 2019
Publication Date:
23 May 2021 (online)
? 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

|?Fig. 1: (a) Juvenile idiopathic arthritis ? peripheral smear shows neutrophilic leukocytosis ? Giemsa, ?100. (b) Reactive marrow and absence of blasts ? Giemsa, ?100

|?Fig. 2: Acute leukemia (a) Peripheral pancytopenia ? Giemsa, ?100. (b) Hypercellular marrow with prominence of blasts ? Giemsa, ?100 (inset shows blasts ? Giemsa, ?400)
References
- 1?Linabery AM, Ross JA.?Trends in childhood cancer incidence in the U.S. (1992-2004). Cancer 2008; 112: 416-32
- 2?de Camargo B, de Oliveira Santos M, Rebelo MS, de Souza Reis R, Ferman S, Noronha CP. et al.?Cancer incidence among children and adolescents in Brazil:First report of 14 population-based cancer registries. Int J Cancer 2010; 126: 715-20
- 3?Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK. et al.?Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 2008; 58: 15-25
- 4?Barr R, Riberio R, Agarwal B, Masera G, Hesseling P, Magrath I.?Pediatric oncology in countries with limited resources. In: Pizzo PA, Poplack DG. editors Principles and Practice of Pediatric Oncology. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2006: 1605-17
- 5?Bashar A.?Incidence and pattern of childhood cancers in India: Findings from population-based cancer registries. Indian J Cancer 2016; 53: 511-2
- 6?Barbosa CM, Nakamura C, Terreri MT, Lee ML, Petrilli AS, Hil?rio MO.?Musculoskeletal manifestations as the onset of acute leukemias in childhood. J Pediatr (Rio J) 2002; 78: 481-4
- 7?Robazzi TC, Barreto JH, Silva LR, Santiago MB, Mendon?a N.?Osteoarticular manifestations as initial presentation of acute leukemias in children and adolescents in Bahia, Brazil. J Pediatr Hematol Oncol 2007; 29: 622-6
- 8?Campos LM, Goldstein S, Santiago RA, Jesus AA, Cristofani LM, Odone Filho V. et al.?Musculoskeletal involvement as a first manifestation of neoplasm disease. Rev Assoc Med Bras (1992) 2008; 54: 132-8
- 9?Schaller J.?Arthritis as a presenting manifestation of malignancy in children. J Pediatr 1972; 81: 793-7
- 10?Saulsbury FT, Sabio H.?Acute leukemia presenting as arthritis in children. Clin Pediatr (Phila) 1985; 24: 625-8
- 11?Goncalves M, Terreri MT, Barbosa CM, Len CA, Lee L, Hilario MO.?Diagnosis of malignancies in children with musculoskeletal complaints. Sao Paulo Med J 2005; 123: 21-3
- 12?Murray MJ, Tang T, Ryder C, Mabin D, Nicholson JC.?Childhood leukaemia masquerading as juvenile idiopathic arthritis. BMJ 2004; 329: 959-61
- 13?Tafaghodi F, Aghighi Y, Rokni Yazdi H, Shakiba M, Adibi A.?Predictive plain X-ray findings in distinguishing early stage acute lymphoblastic leukemia from juvenile idiopathic arthritis. Clin Rheumatol 2009; 28: 1253-8
- 14?Marwaha RK, Kulkarni KP, Bansal D, Trehan A.?Acute lymphoblastic leukemia masquerading as juvenile rheumatoid arthritis: Diagnostic pitfall and association with survival. Ann Hematol 2010; 89: 249-54
- 15?Lovell DJ.?Juvenile idiopathic arthritis: Clinical features. In: Kippel JH, Stone JH, Crofford LJ, White PH. editors Primer on the Rheumatic Diseases. 13th ed. New York: Springer Science; 2008
- 16?Simard JF, Neovius M, Hagelberg S, Askling J.?Juvenile idiopathic arthritis and risk of cancer: A nationwide cohort study. Arthritis Rheum 2010; 62: 3776-82
- 17?Yanagimachi M, Miyamae T, Naruto T, Hara T, Kikuchi M, Hara R. et al.?Association of HLA-A*02:06 and HLA-DRB1*04:05 with clinical subtypes of juvenile idiopathic arthritis. J Hum Genet 2011; 56: 196-9
- 18?Tamashiro MS, Aikawa NE, Campos LM, Cristofani LM, Odone-Filho V, Silva CA.?Discrimination of acute lymphoblastic leukemia from systemic-onset juvenile idiopathic arthritis at disease onset. Clinics (Sao Paulo) 2011; 66: 1665-9
- 19?Brix N, Rosth?j S, Herlin T, Hasle H.?Arthritis as presenting manifestation of acute lymphoblastic leukaemia in children. Arch Dis Child 2015; 100: 821-5
- 20?Mulder H, Herregods N, Mondelaers V, Benoit Y, De Moerloose B.?Musculoskeletal manifestations in children with acute lymphoblastic leukaemia. Belg J Hematol 2012; 3: 3-11
- 21?Jali S, Prashanth GP, Amarkhed P.?Migratory polyarthritis in aleukemic lymphoblastic leukemia: An undesignated paraneoplastic syndrome. J Sci Soc 2013; 40: 44-6
- 22?Wallendal M, Stork L, Hollister JR.?The discriminating value of serum lactate dehydrogenase levels in children with malignant neoplasms presenting as joint pain. Arch Pediatr Adolesc Med 1996; 150: 70-3
- 23?Jones OY, Spencer CH, Bowyer SL, Dent PB, Gottlieb BS, Rabinovich CE.?A multicenter case-control study on predictive factors distinguishing childhood leukemia from juvenile rheumatoid arthritis. Pediatrics 2006; 117: e840-4


 PDF
PDF  Views
Views  Share
Share

