ABVE-PC and modified BEACOPP regimen in Indian children with Hodgkin lymphoma: Feasibility and efficacy
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(02): 106-111
DOI: DOI: 10.4103/0971-5851.180142
Abstract
Aims: To study the toxicity of ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone and cyclophosphamide) and modified-BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide,vincristine, procarbazine, prednisone) in intermediate-risk and high-risk Hodgkin lymphoma patients. Methods: High-risk patients received 4 cycles of modified-BEACOPP (m-BEACOPP) plus 4 cycles of ABVD. Intermediate-risk patients received 4 cycles of ABVE-PC plus 2 cycles of ABVD. Results: From 2010 to 2014, 17 patients received 66 cycles of m-BEACOPP and 9 patients received 40 cycles of ABVE-PC. In the m-BEACOPP and ABVE-PC courses, respectively, significant thrombocytopenia (< 50,000/mm3) occurred in 10.6% vs 0% of courses; anemia (Hb. < 8 gm/dl) in 27.3% vs 15%; neutropenia (ANC < 500/mm3) in 46.9% vs 32.5%; and febrile neutropenia in 33.3% vs. 22.5%. Only episode of documented infection (hepatic abscess) occurred in ABVE-PC. There were no episodes of sepsis, typhlitis or pneumonia in either group. All 26 patients are in remission with a median follow-up of 35 months (range, 17-61); and there have been no relapses. Two of 26 (7.7%) patients failed to achieve rapid early response after 2 cycles and complete remission after 4 cycles of chemotherapy; both achieved remission with more intensive regimens followed by radiation. The remaining 24 patients did not receive radiation therapy. Conclusions: Both m-BEACOPP and ABVE-PC regimens have acceptable toxicity; and thus can be used in most centres with optimum supportive care facilities. They offer promising response rate and relapse free survival without the need for radiation therapy in most patients; and thus may be considered for children with high-risk and intermediate-risk Hodgkin lymphoma.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Aims:
To study the toxicity of ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone and cyclophosphamide) and modified-BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) in intermediate-risk and high-risk Hodgkin lymphoma patients.
Methods:
High-risk patients received 4 cycles of modified-BEACOPP (m-BEACOPP) plus 4 cycles of ABVD. Intermediate-risk patients received 4 cycles of ABVE-PC plus 2 cycles of ABVD.
Results:
From 2010 to 2014, 17 patients received 66 cycles of m-BEACOPP and 9 patients received 40 cycles of ABVE-PC. In the m-BEACOPP and ABVE-PC courses, respectively, significant thrombocytopenia ( < 50,000/mm3) occurred in 10.6% vs 0% of courses; anemia (Hb. < 8 gm/dl) in 27.3% vs 15%; neutropenia (ANC < 500/mm3) in 46.9% vs 32.5%; and febrile neutropenia in 33.3% vs. 22.5%. Only episode of documented infection (hepatic abscess) occurred in ABVE-PC. There were no episodes of sepsis, typhlitis or pneumonia in either group. All 26 patients are in remission with a median follow-up of 35 months (range, 17-61); and there have been no relapses. Two of 26 (7.7%) patients failed to achieve rapid early response after 2 cycles and complete remission after 4 cycles of chemotherapy; both achieved remission with more intensive regimens followed by radiation. The remaining 24 patients did not receive radiation therapy.
Conclusions:
Both m-BEACOPP and ABVE-PC regimens have acceptable toxicity; and thus can be used in most centres with optimum supportive care facilities. They offer promising response rate and relapse free survival without the need for radiation therapy in most patients; and thus may be considered for children with high-risk and intermediate-risk Hodgkin lymphoma.
INTRODUCTION
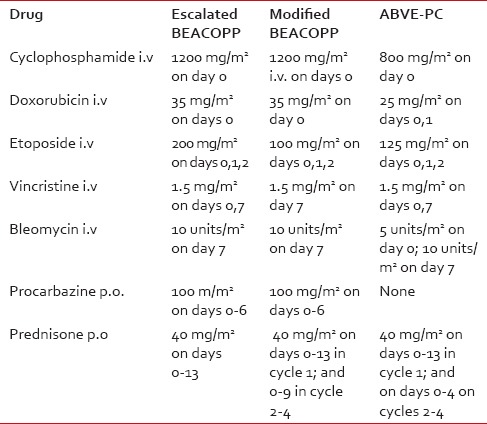
Hodgkin lymphoma (HL) ranks among the malignancies most curable with chemotherapy, alone or in combination with radiation, in both children and adults. The doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) regimen, introduced in 1976,[1] has been one of the most widely used regimens because of its efficacy in advanced stage HL[2] and its low-risk of gonadal toxicity and secondary cancers.[3,4] Subsequent regimens introduced to further improve the cure rate of HL include cyclophosphamide, vincristine, procarbazine, prednisone (COPP)-ABVD,[5] COPP-ABV,[6] bleomycin, etoposide, doxorubicin, COPP (BEACOPP),[7] and doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC).[8] [Table 1 for drug combinations for the various regimens]. In a Children's Oncology Group (COG) study of children with high-risk HL, the escalated BEACOPP (e-BEACOPP) produced a 5-year event-free survival (EFS) of 94% and an overall (OS) of 97%.[9] In another study, the ABVE-PC regimen had an EFS of 84% in high-risk patients and 85% in intermediate-risk patients.[8] Available studies from India indicate ABVD, COPP-ABVD, and COPP-ABV are the regimens commonly used in Indian children;[10,11,12,13,14] but there is no data on the use of ABVE-PC or BEACOPP. Herein, we report our limited experience with these two regimens in Indian children with intermediate and high-risk HL respectively, and review the literature on the efficacy of these regimens in children.
Table 1
Drugs and dosages for various regimens
PATIENTS AND METHODS
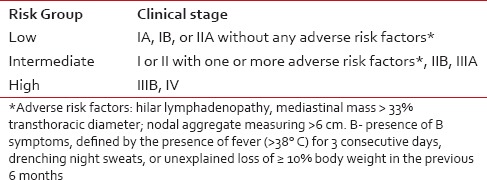
Beginning March 2010, at a newly established Pediatric Cancer Center at Meenakshi Mission Hospital and Research Centre (MMHRC), Madurai, India, HL patients were treated as follows: High-risk patients received 4 cycles of modified-BEACOPP (m-BEACOPP) plus 4 cycles of ABVD. Among the intermediate-risk patients, first two patients received 6 cycles of ABVE-PC; all other patients received 4 cycles of ABVE-PC plus 2 cycles of ABVD. The risk classification is outlined in Table 2. Our m-BEACOPP regimen differs from the escalated BEACOPP in two aspects: The etoposide dose is reduced to 100 mg/m2 daily ×3 days and the duration of prednisone therapy from 14 to 10 days, except for the first course [Table 1]. In mid-2012, a second center, Kanchi Kamakoti Childs Trust Hospital (KKCTC), Chennai, India, joined us in the use of m-BEACOPP for high-risk HL patients and has treated 7 consecutive patients. All HL patients consecutively seen and treated at MMHRC were included in the study. Two patients (both from MMHRC) died before starting treatment — one from respiratory arrest due to mediastinal mass during biopsy; another from congestive cardiac failure due to long-standing severe anemia. These two patients are not included in the analysis since the purpose of the study is to evaluate the toxicity and efficacy of the two regimens.
Table 2
Risk stratification of patients
During the past 4½ years, we have treated a total of 17 high-risk patients and 9 intermediate-risk patients, for a total of 26 patients. The demographics of the patients: Median age of 9 years (range, 4-18); male: female, 21:5. For the administration of first 3 days of chemotherapy in both regimens, the patients were hospitalized for three days; Patients on both regimens received the first 3 days of their chemotherapy as inpatients, and their day 7 therapy in the outpatient Infusion Center (Day Care).
Response to therapy was assessed by follow-up computed tomography (CT) scan after the completion of Cycle 2; and if necessary, after Cycle 4. The approximate volume of the mass was calculated by the following formula: Tumor volume in cm3 = a × b × c × 0.52 (a, b, and c are the anteroposterior transverse and vertical dimensions of the tumor).[15] A rapid early response (RER) was defined as ≥70% reduction in the tumor volume at the completion of two cycles of therapy; and a complete remission (CR) was defined as complete disappearance of the mass or ≥90% reduction on CT imaging (in the absence of fluorodeoxyglucose positron emission tomography [FDG-PET] scan) or PET negativity, when available. FDG-PET scan was done only on the 7 patients seen at the second center, KKCTC.
For monitoring pulmonary toxicity from bleomycin, patients were routinely questioned for shortness of breath on exertion and about their exercise tolerance; and all patients either underwent a high-resolution CT scan of the chest (for evidence of pulmonary fibrosis) or pulmonary function tests (PFT) afer 4 cycles of treatment, depending upon the availability. Upon completion of treatment, all patients were followed every 3 months until 3 years from diagnosis; and less frequently thereafter. For monitoring of cardiac toxicity, the echocardiogram was repeated at the end of the treatment and 3 years later.
The cost of treatment was estimated by calculating the cost for a hypothetical patient weighing approximately 30 kg (with a surface area of 1 m2) — by adding the cost of the following three components:
- The cost of drugs used for each course × the number of courses;
- The cost of 3 days of hospitalization for chemotherapy; and
- The cost of 1 day of hospitalization for the management of febrile neutropenia × the expected average number of days hospitalizations for the regimen.
RESULTS
Toxicity
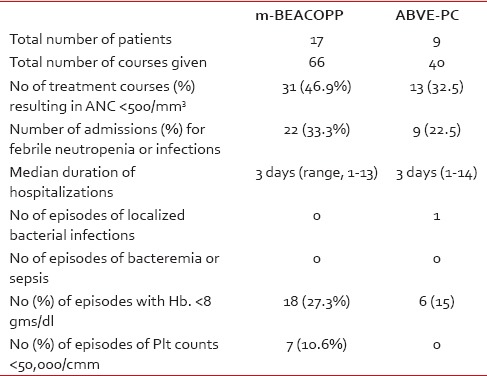
Overall, m-BEACOPP therapy resulted in more episodes of significant neutropenia (absolute neutrophil count [ANC], < 500), anemia (hemoglobin < 8 g/dl), thrombocytopenia (platelet count, < 50,000/uL), and more hospitalizations for febrile neutropenia, compared to the ABVE-PC therapy; but the only episode of documented bacterial infection (hepatic abscess with complete recovery) occurred following ABVE-PC. There were no episodes of bacteremia, sepsis, typhlitis, or pneumonia in either group. More details about the incidence of cytopenias are provided in Table 3.
Table 3
Toxicity of m-BEACOPP and ABVE-PC
No patient had any clinical symptoms or signs of cardiac toxicity. None of the 19 patients who had either PFT or chest CT had evidence of pulmonary toxicity; and all 24 patients who had follow-up echocardiogram had normal studies with an ejection fraction of ≥60%. There were no cases of second malignancies thus far with a median follow-up of 35 months (range, 17-61).
Cost of treatment
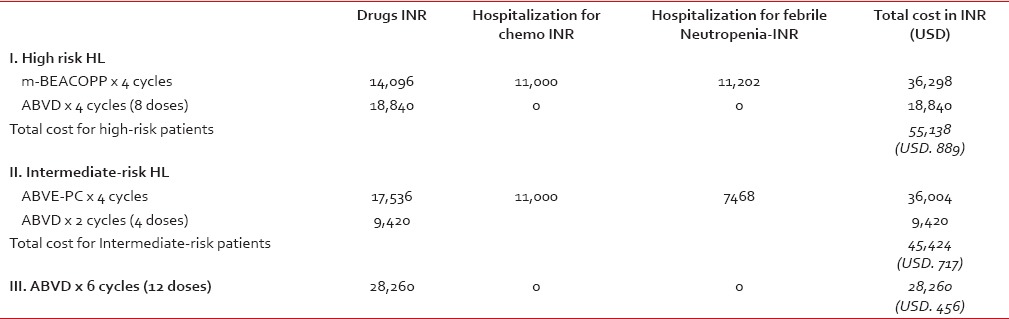
The cost of treating a hypothetical child (weighing about 30 kg, and with a surface area of 1 m2) for Hodgkin' disease was estimated to be: INR. 55138 (USD. 889) for high-risk patients receiving 4 cycles of m-BEACOPP and 4 cycles of ABVD; and INR. 44,424 (USD. 717) for intermediate-risk patients receiving 4 cycles of ABVE-PC and 2 cycles of ABVD. For comparison, the cost for 6 cycles (12 doses) of ABVD as outpatient was estimated to be INR. 28,260 (USD. 456). The ABVD courses did not incur any expenses for the administration of chemotherapy, and rarely required hospitalizations for febrile neutropenia. The breakdown figures for each of the three items contributing to the cost (drugs, hospitalization for chemotherapy, and hospitalization for febrile neutropenia) are given in Table 4.
Table 4
Cost of therapy for various treatment groups
Outcome
Twenty-four of 26 patients (92.3%) showed RER after 2 cycles of treatment and achieved CR after 4 cycles. The 2 patients who did not achieve an RER at the end of 2 cycles failed to achieve CR at the end of 4 cycles. However, both achieved CR with further courses of more intensive chemotherapy, followed by involved-field radiation therapy (IFRT). These salvage regimens included ICE (ifosfamide, carboplatin, etoposide) and/or DECAL (dexamethasone, etoposide, cisplatin, high-dose cytarabine and L-asparaginase). All 26 patients are alive and doing well with a relapse-free survival (RFS) and EFS of 100%; all are being followed as outpatients with a median duration of follow-up of 35 months (17-61). No patient has had clinically significant cardiac or pulmonary dysfunction.
DISCUSSION
Our study shows that the m-BEACOPP and ABVE-PC regimens are well tolerated by Indian children, with acceptable toxicity; and they offer a high rate of CR and a low-risk of relapse. Although grade 4 neutropenia (ANC < 500/mm3) was seen in 47% and 31.7%, respectively, of the m-BEACOPP and ABVE-PC courses, no patient developed bacteremia, sepsis, typhlitis, or pneumonia; and none required platelet transfusions. The ABVE-PC regimen was overall less myelotoxic, although the only case of documented bacterial infection (hepatic abscess) occurred in this group. Twenty-one of 24 (92.3%) patients achieved RER after 2 cycles and CR after 4 cycles of chemotherapy. No patient has relapsed so far with a median follow-up of 35 months (range, 17-61); and none developed any clinically significant pulmonary or cardiac toxicity.
The e-BEACOPP regimen was developed in 1990s to improve upon COPP-ABVD by substituting etoposide for dacarbazine and vinblastine.[5] In a COG study of children with high-risk HL, e-BEACOPP resulted in a high 5-year EFS of 94% and OS of 97%.[9] However, it was associated with significant toxicity including infections in 35% and typhlitits in 4%. In addition, e-BEACOPP is associated with a 6.5% cumulative incidence of second malignancy in adults.[16] In contrast, the m-BEACOPP we used was well-tolerated by our patients, without any incidence of sepsis or typhlitis — probably because of the reduction in the dose of etoposide by 50% and the reduction in the duration of prednisone therapy from 14 to 10 days.
The ABVE-PC regimen was developed as an improvement over ABVD by substituting vincristine for vinblastine to reduce myelotoxicity, and etoposide for dacarbazine to avoid delay in therapy, and by adding prednisone and cyclophosphamide to enhance efficacy.[8] This regimen has lower doses of etoposide than in e-BEACOPP and does not have the alkylating agent procarbazine. Although COG results reported an 8.4% incidence for both sepsis and typhlitis, our patients tolerated this regimen quite well, with only one of 41 courses complicated by a bacterial infection (hepatic abscess), which was treated successfully.
In adults with advanced-stage HL, the chemotherapy regimens that have shown the best outcome include ABVD and e-BEACOPP, often in combination with radiation therapy to bulky disease.[17,18,19,20,21] ABVD has produced a failure-free survival (FFS) of 61-74% at a median follow-up of 5-7 years.[16,17,18,19,20] In a comparative study of ABVD versus e-BEACOPP from Italy, the e-BEACOPP offered a better rate of freedom from first progression (FFS) at 7 years than ABVD (85% vs. 73%, P = 0.004); however, the 7-year OS, after salvage therapy, was not significantly better with e-BEACOPP (89% vs. 84%, P = 0.39).[17] The e-BEACOPP regimen has also been shown to be superior to COPP-ABVD in a randomized trial of adults with intermediate and high-risk HL — with 5-year FFS and OS of 87% and 91%, respectively, for e-BEACOPP, compared to 69% and 83% for COPP-ABVD.[20]
ABVD, COPP-ABVD, and COPP-ABV are the most commonly used regimens in Indian children; and the studies using these regimens are listed in Table 5.[10,11,12,13,14] The EFS reported in these studies range from 50% to 74%; and the OS from 78% to 100%. The best results of 78


 PDF
PDF  Views
Views  Share
Share

