A review of the systemic adverse effects of areca nut or betel nut
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(01): 3-9
DOI: DOI: 10.4103/0971-5851.133702
Abstract
Areca nut is widely consumed by all ages groups in many parts of the world, especially south-east Asia. The objective of this review is to systematically review and collate all the published data that are related to the systemic effects of areca nut. The literature search was performed by an electronic search of the Pubmed and Cochrane databases using keywords and included articles published till October 2012. We selected studies that covered the effect of areca nut on metabolism, and a total of 62 studies met the criteria. There is substantial evidence for carcinogenicity of areca nut in cancers of the mouth and esophagus. Areca nut affects almost all organs of the human body, including the brain, heart, lungs, gastrointestinal tract and reproductive organs. It causes or aggravates pre-existing conditions such as neuronal injury, myocardial infarction, cardiac arrhythmias, hepatotoxicity, asthma, central obesity, type II diabetes, hyperlipidemia, metabolic syndrome, etc. Areca nut affects the endocrine system, leading to hypothyroidism, prostate hyperplasia and infertility. It affects the immune system leading to suppression of T-cell activity and decreased release of cytokines. It has harmful effects on the fetus when used during pregnancy. Thus, areca nut is not a harmless substance as often perceived and proclaimed by the manufacturers of areca nut products such as Pan Masala, Supari Mix, Betel quid, etc. There is an urgent need to recognize areca nut as a harmful food substance by the policy makers and prohibit its glamorization as a mouth freshener. Strict laws are necessary to regulate the production of commercial preparations of areca nut.
Publication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Areca nut is widely consumed by all ages groups in many parts of the world, especially south-east Asia. The objective of this review is to systematically review and collate all the published data that are related to the systemic effects of areca nut. The literature search was performed by an electronic search of the Pubmed and Cochrane databases using keywords and included articles published till October 2012. We selected studies that covered the effect of areca nut on metabolism, and a total of 62 studies met the criteria. There is substantial evidence for carcinogenicity of areca nut in cancers of the mouth and esophagus. Areca nut affects almost all organs of the human body, including the brain, heart, lungs, gastrointestinal tract and reproductive organs. It causes or aggravates pre-existing conditions such as neuronal injury, myocardial infarction, cardiac arrhythmias, hepatotoxicity, asthma, central obesity, type II diabetes, hyperlipidemia, metabolic syndrome, etc. Areca nut affects the endocrine system, leading to hypothyroidism, prostate hyperplasia and infertility. It affects the immune system leading to suppression of T-cell activity and decreased release of cytokines. It has harmful effects on the fetus when used during pregnancy. Thus, areca nut is not a harmless substance as often perceived and proclaimed by the manufacturers of areca nut products such as Pan Masala, Supari Mix, Betel quid, etc. There is an urgent need to recognize areca nut as a harmful food substance by the policy makers and prohibit its glamorization as a mouth freshener. Strict laws are necessary to regulate the production of commercial preparations of areca nut.
INTRODUCTION
Areca nut is the seed Areca catechu, and it grows in much of the tropical Pacific, Asia and parts of East Africa. It is also called as betel nut and is often chewed wrapped inside betel leaves (paan) or with tobacco (betel quid), the composition of which varies in different populations and countries.[1,2] It is one of the most widely consumed addictive substances in the world after nicotine, ethanol and caffeine, and is consumed by approximately 10% of the world's population. Many reports suggest that chewing areca nut starts at a young age, and it is being consumed freely by children.[3,4] The users of areca nut believe that it is helpful for the digestive system and has mild euphoric effects.[3] Areca nut has wide-ranging effects on the human body. It is associated with central obesity and type II diabetes.[3] The IARC review concluded that areca nut is carcinogenic in humans and that it is linked to cancers of the oral cavity, pharynx, esophagus, liver and billary tracts and the uterus.[1] The effects of areca nut are diverse and can be compared with those of the other widely used addictive substances. The knowledge about areca nut and its effects is increasing very rapidly as more and more researches are being published.[3]
MATERIALS AND METHODS
A systematic search of all the relevant literature was performed with keywords such as Non cigarette tobacco products, Carcinogens, Illegal tobacco products, Public policy, Toxicology. The literature search was performed using the Pubmed and Cochrane databases on articles published till October 2012. All the well-designed original studies published in English that covered the effect of areca nut on metabolism were selected. The literature was also enhanced by articles obtained as cross references from the bibliography of the selected articles. There have been lots of articles published on the effects of areca nut on a single organ system or a cellular pathway, but literature showing the effect of areca nut on the human body as a whole is deficient. The aim of this article is to review and highlight the effects of areca nut on all the major organs and systems of the human body.
SYSTEMIC EFFECTS OF ARECA NUT
Metabolism of areca nut
There are four main alkaloids of areca nut, namely arecoline, arecaidine, guvacoline and guvacine [Figure 1]. The major parasympathetic and muscarinic effects of areca nut are due to arecoline. It has been presumed that both arecoline and arecaidine undergo glutathione conjugation as they form mercepturic acid in rats. The major metabolite of arecoline is arecoline 1-oxide. The main mode of arecoline metabolism appears to be hydrolysis to arecaidine and N-oxidation combined with double bond reduction of the arecaidine.[5,6] The N-oxidation of arecoline to arecoline 1-oxidase takes place by flavin-containing monooxygenases 1, flavin containing monooxygenases 3 and not by P-450, which strongly suggests that this reaction may occur in tissues other than the liver, especially the kidneys, where flavin-containing monooxygenases 1 is found in abundance.[6] This points to a possible role of the kidneys in the metabolism and toxicology of arecal alkaloids.[6] The urinary metabolites of arecoline 1-oxidase are 50% arecoline 1-oxidase itself, 30% mercepturic acids and their catabolic products and the remaining 20% are its N-oxide derivatives.[6] A number of nitrosamines are also formed from the arecal alkaloids in the mouth, which play an important role in the causation of oral cancer, especially methylnitrosaminoproprionitrile, which is the most carcinogenic among them.[3,5,6] We hereby summarize the systemic effects of areca nut.
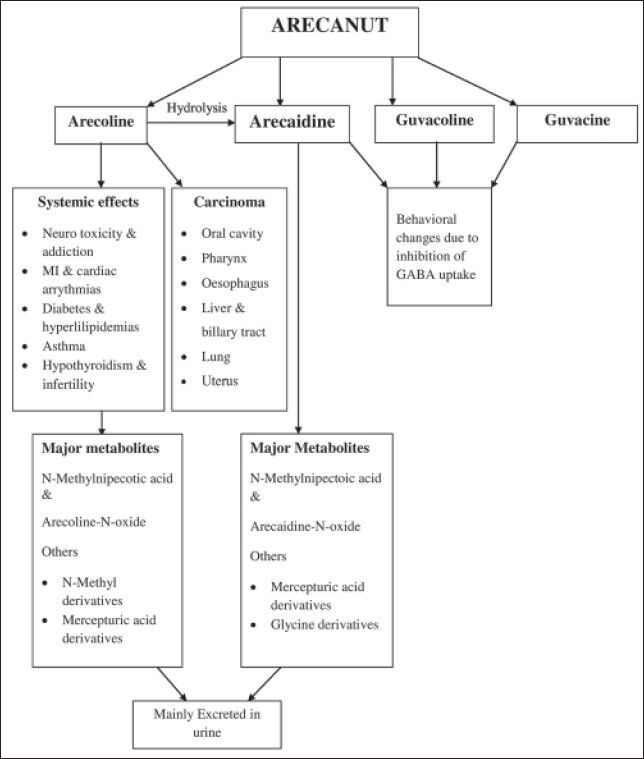
| Figure 1:Major alkaloids of areca nut
Effect on the nervous system
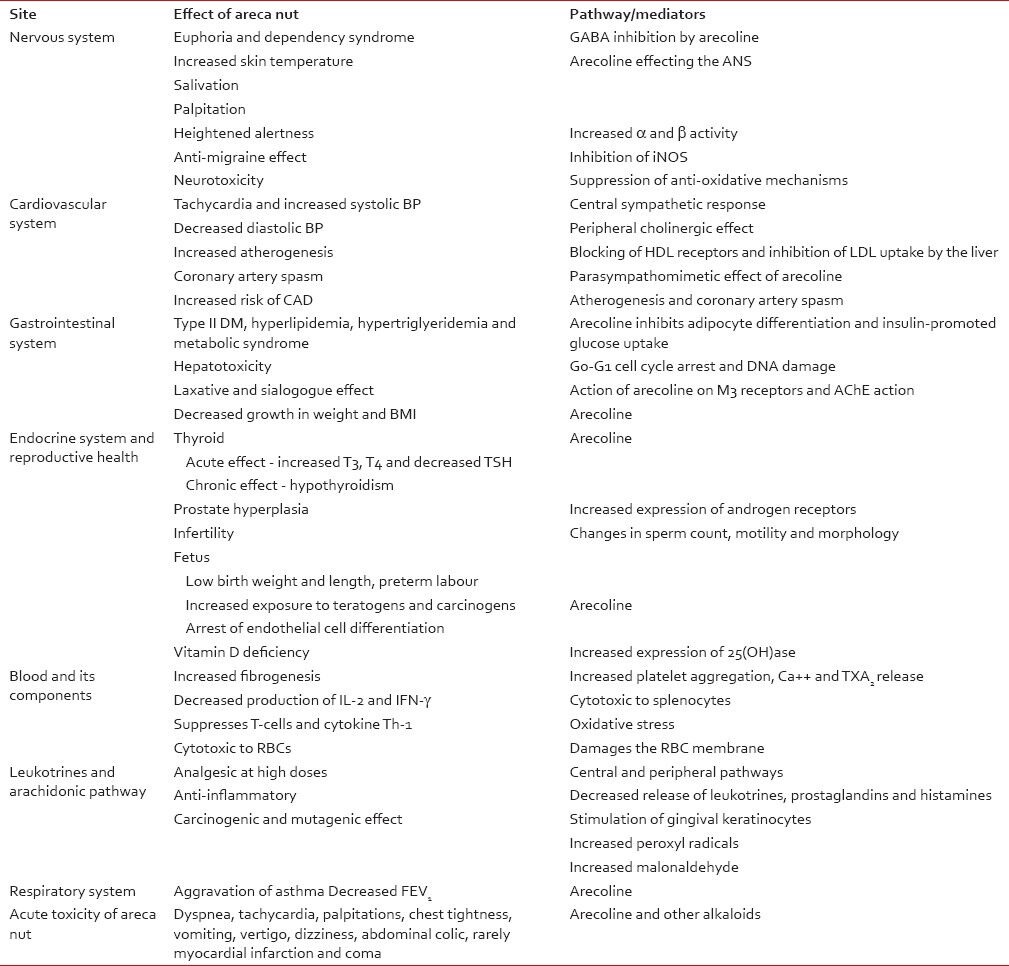
The effects of areca nut are mainly on the central and the autonomic nervous systems due to the alkaloid arecoline, which possesses parasympathomimetic properties stimulating both muscarinic and nicotinic receptors. Habitual users claim euphoria, a sense of well being, warmth, increased alertness, salivation, palpitation, anti-migraine and enhanced capability to work [Table 1].[7] Areca nut use is associated with a dependency syndrome, which comprises increased concentration, mild euphoria, relaxation, postprandial satisfaction and a withdrawal syndrome associated with insomnia, mood swings, irritability and anxiety, the severity of which can be compared with that of amphetamine use.[5] Areca nut leads to palpitation, increased blood pressure, increased body temperature, flushing and sweating within minutes of consumption.[8] Significant slowing of prospective estimation of time intervals is noted with a decrease in choice reaction time, but without any effect on simple reaction time.[9] Contrary to the popular belief, there is no significant effect on memory and concentration is actually decreased.[9] A study comprising recording of EEG of 52 subjects before and after areca nut consumption showed that areca nut consumption caused α and β activity to increase with decreased θ activity. The changes in α activity are seen more in the occipital region, with more global changes in β and θ activity, which is consistent with a state of arousal and some degree of relaxation.[7] There is no evidence to suggest that processing of visual information is facilitated by areca nut, but some peripheral stimulation may occur.[10] Animal studies show that there is inhibition by areca nut of the enzyme iNOS, leading to decreased protein extravasation from the vessels, which explains its anti-migraine use by village folks in India.[11] Arecoline, arecaidine, guvacine and guvacoline cause inhibition of neurosuppressive activity of gama-aminobutyric acid (GABA) by blocking the receptors and inhibiting the uptake[12] thus contributing to the euphoric effect and making a person resistant to benzodiazapines and predisposing to seizures.[12] The schizophrenic users of areca nut show a decrease in both negative and positive symptoms and avoid other harmful recreational drugs and may have severe extrapyramidal symptoms on heavy usage due to arecoline, which has an antagonistic action to procycladine (an anti-cholinergic).[13] Arecoline in concentrations above 50 μM has been shown to cause neuronal injury by causing an increase in oxidative stress and suppression of the anti-oxidant system of the nervous system, and, at higher concentrations, may cause cell death.[14]
Table 1
Summary of the systemic effects of areca nut
|
Effect on the cardiovascular system
Studies have confirmed that there is an increase in facial temperature by 0.5-2° C rapidly on consumption of areca nut. Areca nut consumption induces adrenal cromaffin cells to release increased catacholamaines [Table 1].[3] A study on 47 subjects showed that areca nut usage resulted in increased heart rate, irrespective of the frequency of usage, due to central sympathetic response, but the effect on blood pressure is more varied, leading to a fall of the diastolic component due to the peripheral cholinergic effect and increase in the systolic component in nonhabitual users. Areca nut does not alter the cerebral blood flow as there is not much significant increase in blood flow of the internal carotid artery and middle cerebral artery.[15,16,17] Arecoline has a blocking effect on the high-density lipoprotein receptor and inhibits the uptake of low-density lipoprotein by the liver thus leading to enhanced atherogenesis and its parasympathomimetic effect leading to spasm of the coronary arteries, both of which predispose to coronary artery diseases.[18,19] Researchers in Taiwan have shown a relative risk of coronary artery disease in areca nut chewers to be 3.5 in men and 1.37 in women, and the risk increases with the amount consumed.[20,21] Studies have also linked areca nut chewing to cardiac arrhythmias like paroxysmal supraventricular tachycardia (PSVT), even causing death in a case despite exhaustive treatment.[22]
Effect on the gastrointestinal system and food metabolism
Areca nut has diverse effects on the digestive system and metabolism of food in the human body. It leads to lowering of plasma cholesterol by up to 25% due to inhibition of intestinal acetyl co-enzyme acyltransferase (ACAT) and pancreatic cholesterol esterase (pACE), resulting in decreased cholesterol absorption[Table 1].[23] Prevalence of type II diabetes, hyperlipidemia, hypertriglyceridemia and metabolic syndromes are more common in areca nut chewers as its metabolite, arecoline, inhibits adipogenic differentiation, induces lipolysis in 3T3-L1 adipocytes and interferes with insulin-promoted glucose uptake.[24,25] Areca nut is hepatotoxic causing mixed type of hepatic injury, which is both cholestatic and hepatocellular, and it increases both serum transaminases and alkaline phosphatase.[26,27] Animal studies have shown that in low doses (up to 0.5 mM), it causes G0-G1 cell cycle arrest and DNA damage and in higher doses (up to 1 mM), it causes apoptosis and necrosis, eventually leading to deranged hepatocyte growth, apoptosis, necrosis, liver cirrhosis and, finally, hepatocellular carcinoma.[26,27] Arecoline neutralizes the effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, a toxic dye) by down-regulation of AhR on human hepatoma cells leading to attenuation of activation of CYP1A1 and thus suggesting a role of arecoline on AhR-mediated metabolism of environmental toxins by the liver.[28] Areca nut chewers have increased gastrointestinal motility due to stimulation of colonic M3 receptors, which is dose dependent, and increased saliva secretion due to the presence of AChE inhibitors and arecoline, leading to laxative and sialogogue effects, respectively, which explains its use in the rural population.[29,30] The increase in body weight and body mass index is slower in areca nut chewers as compared with nonchewers.[31] A study on 458 rural subjects in the UK showed that areca nut chewers had a higher resting metabolic rate (effect seen more in males than females) by about 7% as compared with nonchewers, and this effect was due to areca nut metabolites that effect the thermoregulatory pathways, altering the thermogenic effects of the meal and also through centrally mediated effects by decreasing appetite for food.[31,32,33]
Effect on the endocrine and reproductive systems
Animal studies have shown that arecoline in acute administration causes an increased release of T3, T4 and suppression of thyroid stimulating hormone (TSH); in large doses, it activates the hypothalamic-pituitary-adrenal (HPA) axis, similar to stress response, and in regular use causes hypothyroidism [Table 1].[34] Following regular areca nut use, the plasma concentration of melatonin decreases and that of serotonin increases. Areca nut causes an increase in testosterone concentration, but, interestingly, this effect is not seen with betel quid usage. Areca nut leads to an increased concentration of sialic acid in the seminal vesicle and fructose in the coagulating gland and increased expression of androgen receptors on prostate leading to hyperplasia and hypertrophy, causing problems of an enlarged prostate.[35,36,37] Areca nut causes significantly decreased sperm motility, sperm count, sperm abnormalities and decreased activity of antioxidant enzymes, and may cause infertility with long-term usage.[38] The salivary levels of female hormones progesterone and estradiol remain unchanged in habitual female chewers of areca nut.[39] Areca nut users have aggravated effects of Vitamin D deficiency due to the powerful effect of increased expression of 25(OH)ase, leading to decreased serum calcitriol as areca nut has an independent effect on 25(OH)ase.[40]
Effect on blood
Areca nut causes platelet aggregation associated with phospholipase C activation, mobilization of Ca++, TXB2, which leads to release of growth factors, and increased fibrogenesis that plays a crucial part in its effects on the oral mucosa and cardiovascular system [Table 1].[41] Areca nut causes increased secretion of TNF-α and interleukin-1β by mononuclear cells, which is time and dose dependent; interestingly, this effect is blunted by curcumin.[42] Areca nut is cytotoxic to splenocytes and it decreases the production of IL-2 and IFN-γ; interestingly, there is no effect on IL-4. Overall, it suppresses the activation of T-cells and production of cytokine Th-1 via increased oxidative stress thus interfering with the immune system.[43] Destruction of important genes like p53 occurs with increased frequency when there is continuous exposure to areca nut, leading to proliferation of cells with damaged DNA and ultimately neoplastic changes.[44] The cytotoxic effects of areca nut can be effectively countered by addition of foods rich in n-acetyl cystine or glutathione (GSH) as it increases the cellular thiol levels that prevent DNA damage.[44] The blood of an habitual areca nut user should be used for transfusion cautiously as it is cytotoxic to RBCs, causing significant changes in morphology, loss of band 3 fraction, decreased osmotic deformability index and membrane sulfhydryl groups.[45]
Effect on arachidonic acid and leukotrines pathways
Areca nut has analgesic, anti-inflammatory and antioxidant properties. The analgesic effect is mediated by affecting both the central and the peripheral pathways, and the effect at a dose of 500 mg/kg is almost equivalent to pentazocine [Table 1].[46] The anti-inflammatory effect is mediated by reduction in release of prostaglandins, leukotrines, histamines, IL-6, IL-1 and decreased expression of COX-2, which are pro-inflammatory, and increased release of IL-4, which is anti-inflammatory.[46] The antioxidant properties are due to the increased concentration of total phenolic contents that help in scavenging free radicals.[46] Areca nut induces increased production of PGE-2 and 6-keto-PGF1β from gingival keratinocytes, IL-1 and decreased intracellular glutathione, which promotes inflammation and may contribute to sub-mucous fibrosis and oral cancer.[47] Prostaglandin endoperoxidase synthatase, an intracellular enzyme induced by areca nut, plays an important role in carcinogenesis by increasing peroxyl radicals, increasing malonaldehyde, which is mutagenic, and activation of carcinogens in extrahepatic tissues.[48]
Effect on the respiratory system
Various case reports from different parts of the world have shown that the areca nut metabolite arecoline causes aggravation of disease in asthamatics by increasing bronchoconstriction in a dose-dependent manner and decreasing the forced expiratory volume in 1 second (FEV1) by 30%; also, the rate of hospitalization is higher in asthamatics who chew areca nut [Table 1].[49,50]
Effect on the fetus
Expectant mothers who consume areca nut have higher incidences of low birth weight, low birth length and preterm births.[51] Areca nut in lower doses causes dilation of the umbilical vessels via eNOS, but with increased doses causes arrest of the endothelial cell differentiation and subsequently dysfunction.[52] Betel quid chewers have a higher concentration of heavy metals like lead, arsenic and cadmium, which when taken by pregnant women is harmful to the fetus.[53] Perinatal exposure to areca nut exposes the fetus to the harmful effects of carcinogens as the activity of –SH enzyme, melandialdehyde level, cytochrome-450 is altered [Table 1].[54]
Acute toxicity
Reports are available that areca nut can cause acute toxic symptoms if taken in increased quantites, leading to dyspnea, tachypnea, tachycardia, palpitations, hypotension, chest tightness, nausea, vomiting, dizziness, abdominal colic and even myocardial infarction and coma, but, in the majority of the cases, the effects are transient and the patients have timely recovery [Table 1].[55]
POLICY ISSUES
India has one of the highest incidences of oral cancer patients in the world. The age-adjusted rate of oral cancer in India is 20/100,000 population, which accounts for more than 30% of the total cases of cancer.[56] Areca nut, with its carcinogenic potential, is a contributor to the disease load. Despite contributing to numerous life-threatening diseases and carcinogenic properties, it is easily available in the country and freely consumed by all age groups. Areca nut is marketed as mouth freshener under several names such as Gutka, Paan masala, Supari mix, etc. While Gutka is a combination of smokeless tobacco and areca nut, Paan Masala/Supari Mix are pure areca nut products. The market turnover of Indian Gutka and Paan masala companies is more than 100 billion rupees, and billions more are spent on marketing.[57] The prevalence of areca nut consumption with or without tobacco is very high in India and is a part of the normal social culture in the society, and is chewed for various reasons. In rural areas, it is consumed by about 34.7% of the males as compared with 32.4% of the females.[58] In urban areas, the consumption rate among males is about 37.8% and in females it is about 29.7%.[59] The use of areca nut products is prevalent in adolescents, where 16.4% use it regularly and 13% use it occasionally.[60] Even the well-educated section of the society is consuming areca nut, where 12.5% do it regularly and 27.5% do it occasionally.[61] It is important to note that consumption is higher in lower socio economic groups who are illiterate and daily wage earners.[62] The Paan masala companies have stepped up their surrogate advertising in the form of mouth fresheners. This is creating a new generation of areca nut chewers in the form of naïve adolescents and youngsters. The Gutka companies had long bypassed the laws and continued their business virtually unhindered for many years. Many state governments in India have taken a positive step by banning the sale and production of Gutka. This ban is now effective in 20 states and three union territories of India. The huge loss of human lives and finances due to the morbidity and mortality caused by areca nut and Paan masala addiction is putting tremendous strain on the economy, and is much more than the revenue generated by this industry. The government needs to set up an areca nut control program. It is high time that stricter laws are made to regulate areca nut consumption and stern instructions are issued to the manufactures to have pictorial warnings on the products.
CONCLUSION
Areca nut is an addictive substance consumed in many parts of the world by people of all the age groups. Apart from being carcinogenic to the oral cavity, pharynx, esophagus, liver and uterus, it has many diverse effects on the human body affecting almost all the organs. The systemic effects of areca nut are mainly due to the principle alkaloid arecoline. Areca nut causes euphoria, increase in heart rate, increased blood pressure, GABA inhibition and damage to neurons, but has no effect on concentration and memory. Areca nut causes hyperlipidemia, vasospasm and cardiac arrhythmias leading to an increased risk of myocardial ischemia. Arecoline interferes with the fat metabolism leading to Type II diabetes, metabolic syndromes and deranged blood lipid levels. Chronic areca nut consumption causes hypothyroidism, prostate hyperplasia, infertility and Vitamin D deficiency. Areca nut interferes with the immune system by interfering with the activation of T-cells and production of cytokines. Areca nut chewers are predisposed to asthma as it causes bronchoconstriction and decreased FEV1. Women who consume areca nut regularly have more incidences of low birth weight and preterm deliveries. Thus, it is evident that areca nut is a harmful and addictive substance that affects the whole human body, and its use must be tightly regulated for the welfare of the society.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Bhisey RA, Boucher BJ, Chen TH, Gajalakshmi V, Gupta PC, Hecht SS, Editors. IARC working group on the evaluation of carcinogenic risk to humans: Betel-quid and Areca-nut chewing and some Areca-nut-derived nitrosamines. Lyon: IARC Pres; 2004.
- Gupta PC, Warnakulasuriya S. Globalepidemiology ofareca nut usage. Addict Biol2002;7:77-83.
- Boucher BJ, Mannan N. Metabolic effects of the consumption of Areca catechu. Addict Biol 2002;7:103-10.
- Oakley E, Demaine L, Warnakulasuriya S. Areca(betel) nut chewing habitamong high school children in the commonwealth of the northernmariana islands (Micronesia). Bull World Health Organ 2005;83:656-60.
- Giri S, Idle JR, Chen C, Zabriskie TM, Krausz KW, Gonzalez FJ. A metabolonomic approach to the metabolism of the areca nut alkaloids arecoline and arecaidine in the mouse. Chem Res Toxicol 2006;19:818-27.
- Giri S, Krausz KW, Idle JR, Gonzalez FJ. The metabolomics of (±)-arecoline 1-oxide in the mouse and its formation by human flavin-containing monooxygenases. BiochemPharmacol 2007;73:561-73.
- Chu NS. Neurological aspects of areca and betel chewing. Addict Biol 2002; 7:111-4.
- Chu NS. Effects of betel chewing on the central and autonomic nervous systems. J Biomed Sci 2001;8:229-36.
- Osborne PG, Chou TS, Shen TW. Characterization of the psychological, physiological and EEG profile of acute betel quid intoxication in naïve subjects. PLOS ONE 2011;6:1-11.
- Frewer LJ. The effect of betelnut on human performance. P N G Med J 1990;33:143-5.
- Bhandare A, Kshirsagar A, VyawahareN, Sharma P, Mohite R. Evaluation of anti-migraine potential of Areca catechu to prevent nitro glycerin-induced delayed inflammation in rat meninges: Possible involvement of NOS inhibition. J Ethnopharmacol 2011;136:267-70.
- Huang Z, Xiao B, Wang X, Li Y, Dang H.Betel nut indulgence as a cause of epilepsy. Seizure 2003;12:406-8.
- Sullivan RJ, Allen JS, Otto C, Tiobech J, Nero K. Effects of chewing betel nut on the symptoms of people with schizophrenia in Palau, Micronesia. Br J Pharmacol 2000;177:174-8.
- Shih YT, Chen PS, Wu CH, Tseng YT, Wu YC, Lo YC. Arecoline, a major alkaloid of the areca nut, causes neurotoxicity through enhancement of oxidative stress and suppression of the antioxidant protective system. Free RadicBiol Med 2010;49:1471-9.
- Lin SK, Chang YJ, Ryu SJ, Chu NS. Cerebral hemodynamic responses to betel chewing: Doppler study. ClinNeuropharmacol 2002;25:244-50.
- Chu NS. Cardiovascular responses to betel chewing. J Formos Med Assoc 1993;92:835-7.
- Chiou SS, Kou CD. Effect of chewing a single betel-quid on autonomic nervous modulation in healthy young adults. J Psychopharmacol 2008;22:910-7.
- Choudhury MD, Chetia P, Choudhury KD, Talukdar AD, Choudhri MD. Atherogenic effect of arecoline:A computational study. Bioinformation 2012;8:229-32.
- ;Hung DZ, Deng JF. Acute myocardial infarction temporarily related to betel nut chewing. Vet Hum Toxicol 1998;40:25-8.
- Tsai WC, Wu MT, Wang GJ, Lee KT, Lee CH, Lu YH, et al. Chewing areca nut increases the risk of coronary artery disease in Taiwanese men: A case control study. BMC Public Health 2012;12:162-8.
- Guh JY, Chen HC, Tsai JF, ChuangLY. Betel-quid use is associated with heart disease in women. Am J ClinNutr 2007;85:1229-35.
- Chiang WT, Yang CC, Deng JF, Bullard M. Cardiac arrhythmia and betel nut chewing-is there a causal effect. Vet Hum Toxicol 1998;40:287-9.
- Park YB, Jeon SM, Byun SJ, Kim HS, Choi MS. Absorption of intestinal free cholesterol is lowered by supplementation of Areca catechu L. extracts in rats. Life Sci 2002;70:1849-59.
- Hsu HS, Tsou TC, Chao HR, Shy CG, Kuo YT, Tsai FY, et al. Effects of arecoline on adipogenesis, lipolysis and glucose uptake of adipocytes-A possible role of betel-quid chewing in metabolic syndrome. ToxicolApplPharmacol 2010;245:370-7.
- Hsieh TJ, Hsieh PC, Wu MT, Chang WC, Hsio PJ, Lin KD, et al. Betel nut extracts and arecoline block insulin signaling and lipid storage in 3T3-L1 adipocytes. Cell BiolToxicol 2011;27:397-411.
- Chou WW, Guh JY, Tsai JF, Hwang CC, Cheng HC, Huang JS, et al. Arecoline induced growth arrest and p21 WAF[1] expression are dependent on p53 in rat hepatocytes. Toxicology 2008;243:1-10.
- Lin CF, Shiau TJ, Ko YC, Chen PH, Wang JD. Prevalence and determinants ofbiochemical dysfunction of the liver in Atayal aboriginal community of Taiwan: Is betel nut chewing a risk factor. BMC Gastroenterol 2008;8;13.
- Chang ES, Miao ZF, Lee WJ, Chao HR, Li LA, Wang YF, et al. Arecoline inhibits the 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced cytochrome P450 1A1 activation in human hepatoma cells. J Hazard Mater 2007;146:356-61.
- Li CB, Yang X, Tang WB, Liu CY, Xie DP. Arecoline excites the contraction of distal colonic smooth muscle strips in rats via the M3 receptor-extracellular Ca2+ influx-Ca2+ store release pathway. Can J PhysiolPharmacol 2010;88:439-47.
- Gilani AH, Ghayur MN, Saify ZS, Ahmed SP, Choudhary MI, Khalid A. Prescence of Cholinomimetic and acetylcholinesterase inhibitory constituents in betel nut. Life Sci 2004;75:2377-89.
- Strickland SS, Duffield AE. Anthropometric status and resting metabolic rate in users of the areca nut and smokers of tobacco in rural Sarawak. Ann Hum Biol 1997;24:453-74.
- Strickland SS, Veena GV, Houghton PJ, Stanford SC, KurpadAV. Areca nut, energy metabolism and hunger in Asian Men. Ann Hum Biol 2003;30:26-52.
- Chiang CP, Chang MC, Lee JJ, Chang JY, Lee PH, Hahn LJ, et al. Hamster chewing betel quid or areca nut directly show a decrease in body weight and survival rates with concomitant epithelial hyperplasia of cheek pouch. Oral Oncol 2004;40:720-7.
- Saha I, Sengupta SC, Nag D. Ultrastructural and hormonal modulations of the thyroid gland following arecoline treatment in albino mice. Mol Cell Endocrinol 2010;319:1-7.
- Saha I, Chatterji U, Sengupta SC, Nag TC, Nag D, Bannerjee S, et al. Ultrastructural and hormonal changes in the pineal-testicular axis following arecoline administration in rats. J ExpZool A Ecol Genet Physiol 2007;307:187-98.
- Saha I, Chatterjee A, Mondal A, Maiti BR, Chtterjee U. Arecoline augments cellular proliferation in the prostate gland of male wistar rats. ToxicolApplPharmacol 2011;255:160-8.
- Yang NY, Kaphle K, Wang PH, Jong DS, Wu LS, Lin JH. Effects of aqueous extracts of betel quid and its constituents on testosterone production by dispersed mouse interstitial cells. Am J Chin Med 2004;32:705-15.
- Wu PF, Chiang TA, Chen MT, Lee CP, Chen PH, Ko AM, et al. A characterization of the antioxidant enzyme activity and reproductive toxicity in male rats following sub-chronic exposure to areca nut extracts. J Hazard Mater 2010;178:541-6.
- Núñez-de la Mora A, Chatterton RT, Mateo ET, Jesmin F, Bentley GR. Effects of chewing betel nut on measurements of salivary progesterone and estradiol. Am J PhysAnthropol 2007;132:311-5.
- Ogunkolade WB, Boucher BJ, Bustin SA, Burrin JM, Noonan K, Mannan N, et al. Vitamin D metabolism in peripheral blood mononuclear cells is influenced by chewing betel nut (Areca Catechu) and Vitamin D status. J ClinEndocrinolMetab 2006;91:2612-7.
- Jeng JH, Chen SY, Liao CH, Tung YY, Lin BR, Hahn LJ, et al. Modulation of platelet aggregation by areca nut and betel leaf ingredients: Role of reactive oxygen species and cyclooxygenase. Free RadicBiol Medic 2002;32:860-71.
- Chang LY, Wan HC, Lai YL, Kuo YF, Liu TY, Chen YT, et al. Areca nut extracts increased expression of inflammatory cytokines, tumour necrosis factor- α, interleukin-1β, interleukin-6 and interleukin-8 in peripheral blood mononuclear cells. J Peridont Res 2009;44:175-83.
- Wanga CC, Liub TY, Weyc SP, Wanga FY, Jana TR. Areca nut extract suppresses T-cell activation and interferon-γ production via the induction of oxidative stress.Food ChemToxicol 2007;45:1410-8.
- Chatterjee A, Deb S. Genotoxic effect of arecoline given either by the peritoneal or oral route in murine bone marrow cells and the influence of N-acetylcysteine. Cancer Lett 1998;139:23-31.
- Peng K, Chiou, Chen Y, Liu TZ. Is the blood donated by habitual nut quid chewers suitable for use in transfusion. J Formos Med Assoc 2010;109:106-12.
- Bhandare AM, Kshirsagar AD, Vyawahare NS, Hadambar AA, Thorva VS. Potential analgesic, anti-inflammatory and antioxidant activities of hydroalcoholic extract of Areca catechu L. nut. Food ChemToxicol 2010;48:3412-7.
- Jeng JH, Ho YS, Chan YC, Wang YJ, Hahn LJ, Lei D, et al. Areca nut extract up-regulates prostaglandin production, cyclooxygenase-2 m RNA and protein expression of human oral keratinocytes. Carcinogenesis2000;21:1365-70.
- Yang CY, Meng CL, Bijl P, Lee HK. The effect of betel nut extract on cell growth and prostaglandin endoperoxide synthase in human epidermoid carcinoma cells. Prostaglandins Other Lipid Mediat 2002;67:181-95.
- Taylor RH, Al-Jarad N, John LM, Barnes NC, Conroy DM. Betel nut chewing and asthma. Lancet 1992;339:1134-6.
- Sekkadde KK, Saweri A. Betel nut chewing causes bronchoconstriction in some asthma patients. P N G Med J 1994;37:90-9.
- Senn M, Baiwog F, Winmai J. Betel nut chewing during pregnancy, Madang province, Papua New Guinea. Drug Alcohol Depend2009;105:126-31
- Kuo FC, Wu DC, Yuan SS, Hsiao KM, Wang YY, Yang YC, et al. Effects of arecoline in relaxing human umbilical vessels and inhibiting endothelial cell growth. J PerinatMed 2005;33:399-405.
- Al-Rmalli SW, Jenkins RO, Haris PI. Betel quid chewing elevates human exposure to arsenic, cadmium and lead. J Hazard Mater 2011;190:69-74.
- Singh A, RaoAR. Effect of areca nut, a masticatory, on hepatic drug metabolizing enzymes -SH content and lipid peroxidation in lactating mothers and their suckling neonates. Cancer Lett 1995;92:175-80.
- Deng JF, Ger J, Tsai WJ, Kao WF, Yang CC. Acute toxicities of betel nut:Rare but probably overlooked events. J ToxicolClinToxicol 2001;39:355-60.
- Coelho KR. Challenges of the Oral Cancer Burden in India.J Cancer Epidemiol 2012;2012:1-17.
- Businessandeconomy.org. New Delhi:Planman Media Pvt Ltd; c2008[updated 2011 May 26; cited 2012 Nov 12]. Avialable from: http://www.businessandeconomy.org/26052011/storyd.asp?sid=6160andpageno=2 [Last accessed on 2012 December 15].
- Daftary DK, Bhonsle RB, MurtiRBl. An oral lichen planus-like lesion in Indian betel-tobacco chewers. Scand J Dent Res 1980;88:244-9.
- Gupta PC. Survey of sociodemographic characteristics of tobacco use among 99 598 individuals in Bombay, India using handheld computers. Tob Control 1996;5:114-20.
- George A, Varghese C, Sankaranarayanan R. Use of tobacco and alcoholic beverages by children and teenagers in a low-income coastal community in South India. J Cancer Educ 1994;9:111-3.
- Sinha DN, Gupta PC. Tobacco and areca nut use in male medical students of Patna. Natl Med J India 2001;14:176-8.
- Hashibe M, Jacob BJ, Thomas G. Socioeconomic status, lifestyle factors and oral premalignant lesions. Oral Oncol 2003;39:664-71.

| Figure 1:Major alkaloids of areca nut
References
- Bhisey RA, Boucher BJ, Chen TH, Gajalakshmi V, Gupta PC, Hecht SS, Editors. IARC working group on the evaluation of carcinogenic risk to humans: Betel-quid and Areca-nut chewing and some Areca-nut-derived nitrosamines. Lyon: IARC Pres; 2004.
- Gupta PC, Warnakulasuriya S. Globalepidemiology ofareca nut usage. Addict Biol2002;7:77-83.
- Boucher BJ, Mannan N. Metabolic effects of the consumption of Areca catechu. Addict Biol 2002;7:103-10.
- Oakley E, Demaine L, Warnakulasuriya S. Areca(betel) nut chewing habitamong high school children in the commonwealth of the northernmariana islands (Micronesia). Bull World Health Organ 2005;83:656-60.
- Giri S, Idle JR, Chen C, Zabriskie TM, Krausz KW, Gonzalez FJ. A metabolonomic approach to the metabolism of the areca nut alkaloids arecoline and arecaidine in the mouse. Chem Res Toxicol 2006;19:818-27.
- Giri S, Krausz KW, Idle JR, Gonzalez FJ. The metabolomics of (±)-arecoline 1-oxide in the mouse and its formation by human flavin-containing monooxygenases. BiochemPharmacol 2007;73:561-73.
- Chu NS. Neurological aspects of areca and betel chewing. Addict Biol 2002; 7:111-4.
- Chu NS. Effects of betel chewing on the central and autonomic nervous systems. J Biomed Sci 2001;8:229-36.
- Osborne PG, Chou TS, Shen TW. Characterization of the psychological, physiological and EEG profile of acute betel quid intoxication in naïve subjects. PLOS ONE 2011;6:1-11.
- Frewer LJ. The effect of betelnut on human performance. P N G Med J 1990;33:143-5.
- Bhandare A, Kshirsagar A, VyawahareN, Sharma P, Mohite R. Evaluation of anti-migraine potential of Areca catechu to prevent nitro glycerin-induced delayed inflammation in rat meninges: Possible involvement of NOS inhibition. J Ethnopharmacol 2011;136:267-70.
- Huang Z, Xiao B, Wang X, Li Y, Dang H.Betel nut indulgence as a cause of epilepsy. Seizure 2003;12:406-8.
- Sullivan RJ, Allen JS, Otto C, Tiobech J, Nero K. Effects of chewing betel nut on the symptoms of people with schizophrenia in Palau, Micronesia. Br J Pharmacol 2000;177:174-8.
- Shih YT, Chen PS, Wu CH, Tseng YT, Wu YC, Lo YC. Arecoline, a major alkaloid of the areca nut, causes neurotoxicity through enhancement of oxidative stress and suppression of the antioxidant protective system. Free RadicBiol Med 2010;49:1471-9.
- Lin SK, Chang YJ, Ryu SJ, Chu NS. Cerebral hemodynamic responses to betel chewing: Doppler study. ClinNeuropharmacol 2002;25:244-50.
- Chu NS. Cardiovascular responses to betel chewing. J Formos Med Assoc 1993;92:835-7.
- Chiou SS, Kou CD. Effect of chewing a single betel-quid on autonomic nervous modulation in healthy young adults. J Psychopharmacol 2008;22:910-7.
- Choudhury MD, Chetia P, Choudhury KD, Talukdar AD, Choudhri MD. Atherogenic effect of arecoline:A computational study. Bioinformation 2012;8:229-32.
- ;Hung DZ, Deng JF. Acute myocardial infarction temporarily related to betel nut chewing. Vet Hum Toxicol 1998;40:25-8.
- Tsai WC, Wu MT, Wang GJ, Lee KT, Lee CH, Lu YH, et al. Chewing areca nut increases the risk of coronary artery disease in Taiwanese men: A case control study. BMC Public Health 2012;12:162-8.
- Guh JY, Chen HC, Tsai JF, ChuangLY. Betel-quid use is associated with heart disease in women. Am J ClinNutr 2007;85:1229-35.
- Chiang WT, Yang CC, Deng JF, Bullard M. Cardiac arrhythmia and betel nut chewing-is there a causal effect. Vet Hum Toxicol 1998;40:287-9.
- Park YB, Jeon SM, Byun SJ, Kim HS, Choi MS. Absorption of intestinal free cholesterol is lowered by supplementation of Areca catechu L. extracts in rats. Life Sci 2002;70:1849-59.
- Hsu HS, Tsou TC, Chao HR, Shy CG, Kuo YT, Tsai FY, et al. Effects of arecoline on adipogenesis, lipolysis and glucose uptake of adipocytes-A possible role of betel-quid chewing in metabolic syndrome. ToxicolApplPharmacol 2010;245:370-7.
- Hsieh TJ, Hsieh PC, Wu MT, Chang WC, Hsio PJ, Lin KD, et al. Betel nut extracts and arecoline block insulin signaling and lipid storage in 3T3-L1 adipocytes. Cell BiolToxicol 2011;27:397-411.
- Chou WW, Guh JY, Tsai JF, Hwang CC, Cheng HC, Huang JS, et al. Arecoline induced growth arrest and p21 WAF[1] expression are dependent on p53 in rat hepatocytes. Toxicology 2008;243:1-10.
- Lin CF, Shiau TJ, Ko YC, Chen PH, Wang JD. Prevalence and determinants ofbiochemical dysfunction of the liver in Atayal aboriginal community of Taiwan: Is betel nut chewing a risk factor. BMC Gastroenterol 2008;8;13.
- Chang ES, Miao ZF, Lee WJ, Chao HR, Li LA, Wang YF, et al. Arecoline inhibits the 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced cytochrome P450 1A1 activation in human hepatoma cells. J Hazard Mater 2007;146:356-61.
- Li CB, Yang X, Tang WB, Liu CY, Xie DP. Arecoline excites the contraction of distal colonic smooth muscle strips in rats via the M3 receptor-extracellular Ca2+ influx-Ca2+ store release pathway. Can J PhysiolPharmacol 2010;88:439-47.
- Gilani AH, Ghayur MN, Saify ZS, Ahmed SP, Choudhary MI, Khalid A. Prescence of Cholinomimetic and acetylcholinesterase inhibitory constituents in betel nut. Life Sci 2004;75:2377-89.
- Strickland SS, Duffield AE. Anthropometric status and resting metabolic rate in users of the areca nut and smokers of tobacco in rural Sarawak. Ann Hum Biol 1997;24:453-74.
- Strickland SS, Veena GV, Houghton PJ, Stanford SC, KurpadAV. Areca nut, energy metabolism and hunger in Asian Men. Ann Hum Biol 2003;30:26-52.
- Chiang CP, Chang MC, Lee JJ, Chang JY, Lee PH, Hahn LJ, et al. Hamster chewing betel quid or areca nut directly show a decrease in body weight and survival rates with concomitant epithelial hyperplasia of cheek pouch. Oral Oncol 2004;40:720-7.
- Saha I, Sengupta SC, Nag D. Ultrastructural and hormonal modulations of the thyroid gland following arecoline treatment in albino mice. Mol Cell Endocrinol 2010;319:1-7.
- Saha I, Chatterji U, Sengupta SC, Nag TC, Nag D, Bannerjee S, et al. Ultrastructural and hormonal changes in the pineal-testicular axis following arecoline administration in rats. J ExpZool A Ecol Genet Physiol 2007;307:187-98.
- Saha I, Chatterjee A, Mondal A, Maiti BR, Chtterjee U. Arecoline augments cellular proliferation in the prostate gland of male wistar rats. ToxicolApplPharmacol 2011;255:160-8.
- Yang NY, Kaphle K, Wang PH, Jong DS, Wu LS, Lin JH. Effects of aqueous extracts of betel quid and its constituents on testosterone production by dispersed mouse interstitial cells. Am J Chin Med 2004;32:705-15.
- Wu PF, Chiang TA, Chen MT, Lee CP, Chen PH, Ko AM, et al. A characterization of the antioxidant enzyme activity and reproductive toxicity in male rats following sub-chronic exposure to areca nut extracts. J Hazard Mater 2010;178:541-6.
- Núñez-de la Mora A, Chatterton RT, Mateo ET, Jesmin F, Bentley GR. Effects of chewing betel nut on measurements of salivary progesterone and estradiol. Am J PhysAnthropol 2007;132:311-5.
- Ogunkolade WB, Boucher BJ, Bustin SA, Burrin JM, Noonan K, Mannan N, et al. Vitamin D metabolism in peripheral blood mononuclear cells is influenced by chewing betel nut (Areca Catechu) and Vitamin D status. J ClinEndocrinolMetab 2006;91:2612-7.
- Jeng JH, Chen SY, Liao CH, Tung YY, Lin BR, Hahn LJ, et al. Modulation of platelet aggregation by areca nut and betel leaf ingredients: Role of reactive oxygen species and cyclooxygenase. Free RadicBiol Medic 2002;32:860-71.
- Chang LY, Wan HC, Lai YL, Kuo YF, Liu TY, Chen YT, et al. Areca nut extracts increased expression of inflammatory cytokines, tumour necrosis factor- α, interleukin-1β, interleukin-6 and interleukin-8 in peripheral blood mononuclear cells. J Peridont Res 2009;44:175-83.
- Wanga CC, Liub TY, Weyc SP, Wanga FY, Jana TR. Areca nut extract suppresses T-cell activation and interferon-γ production via the induction of oxidative stress.Food ChemToxicol 2007;45:1410-8.
- Chatterjee A, Deb S. Genotoxic effect of arecoline given either by the peritoneal or oral route in murine bone marrow cells and the influence of N-acetylcysteine. Cancer Lett 1998;139:23-31.
- Peng K, Chiou, Chen Y, Liu TZ. Is the blood donated by habitual nut quid chewers suitable for use in transfusion. J Formos Med Assoc 2010;109:106-12.
- Bhandare AM, Kshirsagar AD, Vyawahare NS, Hadambar AA, Thorva VS. Potential analgesic, anti-inflammatory and antioxidant activities of hydroalcoholic extract of Areca catechu L. nut. Food ChemToxicol 2010;48:3412-7.
- Jeng JH, Ho YS, Chan YC, Wang YJ, Hahn LJ, Lei D, et al. Areca nut extract up-regulates prostaglandin production, cyclooxygenase-2 m RNA and protein expression of human oral keratinocytes. Carcinogenesis2000;21:1365-70.
- Yang CY, Meng CL, Bijl P, Lee HK. The effect of betel nut extract on cell growth and prostaglandin endoperoxide synthase in human epidermoid carcinoma cells. Prostaglandins Other Lipid Mediat 2002;67:181-95.
- Taylor RH, Al-Jarad N, John LM, Barnes NC, Conroy DM. Betel nut chewing and asthma. Lancet 1992;339:1134-6.
- Sekkadde KK, Saweri A. Betel nut chewing causes bronchoconstriction in some asthma patients. P N G Med J 1994;37:90-9.
- Senn M, Baiwog F, Winmai J. Betel nut chewing during pregnancy, Madang province, Papua New Guinea. Drug Alcohol Depend2009;105:126-31
- Kuo FC, Wu DC, Yuan SS, Hsiao KM, Wang YY, Yang YC, et al. Effects of arecoline in relaxing human umbilical vessels and inhibiting endothelial cell growth. J PerinatMed 2005;33:399-405.
- Al-Rmalli SW, Jenkins RO, Haris PI. Betel quid chewing elevates human exposure to arsenic, cadmium and lead. J Hazard Mater 2011;190:69-74.
- Singh A, RaoAR. Effect of areca nut, a masticatory, on hepatic drug metabolizing enzymes -SH content and lipid peroxidation in lactating mothers and their suckling neonates. Cancer Lett 1995;92:175-80.
- Deng JF, Ger J, Tsai WJ, Kao WF, Yang CC. Acute toxicities of betel nut:Rare but probably overlooked events. J ToxicolClinToxicol 2001;39:355-60.
- Coelho KR. Challenges of the Oral Cancer Burden in India.J Cancer Epidemiol 2012;2012:1-17.
- Businessandeconomy.org. New Delhi:Planman Media Pvt Ltd; c2008[updated 2011 May 26; cited 2012 Nov 12]. Avialable from: http://www.businessandeconomy.org/26052011/storyd.asp?sid=6160andpageno=2 [Last accessed on 2012 December 15].
- Daftary DK, Bhonsle RB, MurtiRBl. An oral lichen planus-like lesion in Indian betel-tobacco chewers. Scand J Dent Res 1980;88:244-9.
- Gupta PC. Survey of sociodemographic characteristics of tobacco use among 99 598 individuals in Bombay, India using handheld computers. Tob Control 1996;5:114-20.
- George A, Varghese C, Sankaranarayanan R. Use of tobacco and alcoholic beverages by children and teenagers in a low-income coastal community in South India. J Cancer Educ 1994;9:111-3.
- Sinha DN, Gupta PC. Tobacco and areca nut use in male medical students of Patna. Natl Med J India 2001;14:176-8.
- Hashibe M, Jacob BJ, Thomas G. Socioeconomic status, lifestyle factors and oral premalignant lesions. Oral Oncol 2003;39:664-71.


 PDF
PDF  Views
Views  Share
Share

