A Rare Case of Acute Hemolytic Anemia in a Patient with Newly Diagnosed Multiple Myeloma: Maintaining a Fine Balance between Occam's Razor and Hickam's Dictum
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(02): 214-218
DOI: DOI: 10.1055/s-0042-1753500
Abstract
Anemia is a common feature in multiple myeloma and is multifactorial. A 52-year-old lady was admitted to our hospital with complaints of fatigue, exertional dyspnea, paresthesia, and a recent-onset confusion state. Fundus examination revealed features of hyperviscosity. The patient received 2 units of packed red blood cell transfusion (PRBC) before the present hospital admission. Laboratory investigations revealed severe anemia and thrombocytopenia. The M-protein was 5.8 g/dL. Bone marrow showed sheets of plasma cells. Immunofixation electrophoresis confirmed the presence of an IgAλ band. FISH was positive for IgH-FGFR3 fusion. The investigations confirmed multiple myeloma R-ISS stage III. The patient was immediately started on CyBorD chemotherapy regimen. The patient had indirect hyperbilirubinemia and symptomatic anemia. Initial testing of the patient's sample showed blood grouping discrepancy with DCT, ICT, and auto control positive. The symptomatic anemia persisted requiring PRC transfusions. Further antibody study revealed the presence of anti-Jka antibody—a warm IgG antibody and cold antibody. Subsequently, the patient received Jka antigen-negative B-positive compatible PRBC transfusions and the hemoglobin normalized. Our patient had transfusion-associated alloimmunization along with hyperviscosity. The timely recognition and early institution of plasmapheresis and myeloma-directed therapy along with transfusion of compatible Jka antigen-negative PRBC lead to rapid improvement.
Keywords
alloimmunization - anti-Jka - cold antibody - multiple myeloma
Publication History
Article published online:
28 November 2022
© 2022. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Malignancy Associated Microangiopathic Hemolytic Anemia and ThrombocytopeniaMansoor C Abdulla, Indian Journal of Medical and Paediatric Oncology, 2018
- Recurrent Infections in a Patient with Multiple MyelomaSanjay Kini, Journal of Health and Allied Sciences NU, 2019
- Hyperammonemia without liver disease as a differential diagnostic problem. Two cases with myelomaA. Horváth, Zeitschrift für Gastroenterologie, 2008
- A Case of Plasma Cell Myeloma with Multilobated and Monocytoid MorphologySneha Kakoty, Asian Journal of Oncology
- ACQUIRED FACTOR X DEFICIENCY IN MULTIPLE MYELOMA:A COMPLETE RESPONSE TO CHEMOTHERAPY.M Kos, Revista Urología Colombiana / Colombian Urology Journal, 1987
- Decoding the pathogenesis of Diamond–Blackfan anemia using single-cell RNA-seq<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Effects of Danggui Wuji granules on 16S rDNA, metagenome, and metabolome in BDS mice<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Decoding lymphomyeloid divergence and immune hyporesponsiveness in G-CSF-primed human bone marrow by single-cell RNA-seq<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Upconverting nanoparticle-containing erythrocyte-sized hemoglobin microgels that generate heat, oxygen and reactive oxygen species for suppressing hypoxic tumor...<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Multi-dimensional single-cell characterization revealed suppressive immune microenvironment in AFP-positive hepatocellular carcinoma<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
Abstract
Anemia is a common feature in multiple myeloma and is multifactorial. A 52-year-old lady was admitted to our hospital with complaints of fatigue, exertional dyspnea, paresthesia, and a recent-onset confusion state. Fundus examination revealed features of hyperviscosity. The patient received 2 units of packed red blood cell transfusion (PRBC) before the present hospital admission. Laboratory investigations revealed severe anemia and thrombocytopenia. The M-protein was 5.8 g/dL. Bone marrow showed sheets of plasma cells. Immunofixation electrophoresis confirmed the presence of an IgAλ band. FISH was positive for IgH-FGFR3 fusion. The investigations confirmed multiple myeloma R-ISS stage III. The patient was immediately started on CyBorD chemotherapy regimen. The patient had indirect hyperbilirubinemia and symptomatic anemia. Initial testing of the patient's sample showed blood grouping discrepancy with DCT, ICT, and auto control positive. The symptomatic anemia persisted requiring PRC transfusions. Further antibody study revealed the presence of anti-Jka antibody—a warm IgG antibody and cold antibody. Subsequently, the patient received Jka antigen-negative B-positive compatible PRBC transfusions and the hemoglobin normalized. Our patient had transfusion-associated alloimmunization along with hyperviscosity. The timely recognition and early institution of plasmapheresis and myeloma-directed therapy along with transfusion of compatible Jka antigen-negative PRBC lead to rapid improvement.
Keywords
alloimmunization - anti-Jka - cold antibody - multiple myeloma
Introduction
Multiple myeloma (MM) is a plasma cell neoplastic disorder characterized by the clonal proliferation of malignant plasma cells.[1] Symptomatic patients present with myeloma-defining events, i.e., CRAB features (hypercalcemia, renal insufficiency, anemia, and bone lytic lesions).[2] Herein, we report a case of IgAλ MM presenting with hyperviscosity and hemolytic anemia.
Case Report
A 52-year-old lady, a resident of Southern India, was admitted to our hospital with complaints of fatigue, exertional dyspnea, and paresthesia involving both her lower limbs for the past month. The patient's past medical history was unremarkable. The patient's son reported that she was confused and had an irrelevant speech for 1 week before the hospital admission. On admission, the patient was conscious, disoriented, and irritable. Her vitals were stable, and she was afebrile. Clinical examination revealed severe pallor, peripheral neuropathy, and skin petechiae over the lower extremities. Fundus examination by ophthalmoscope revealed dilated and tortuous retinal veins, and disc edema bilaterally suggestive of hyperviscosity.[3] The patient did not have acrocyanosis, Raynaud's phenomenon, or skin changes. The patient received 2 units of packed red cell (PRC) transfusion before the current hospital admission. Laboratory investigations revealed hemoglobin 3.5 g/dL (normal range: 12–17), WBC 7000/mm3 (normal range, 4000–10000), platelet count of 31,000/mm3 (normal range, 150,000–400,000/mm3), blood urea 71 mg/dL (normal range, 15–45), serum creatinine 1.4 mg/dL (normal range, 0.7–1.5), and raised serum calcium 12.7 meq/L (normal range, 8.4–10.2). The peripheral smear revealed normocytic normochromic RBCs, reduced RBC density, occasional plasma cells, and reduced platelets. There was no evidence of hemolysis, viz., RBC fragments or spherocytes. CT of the chest revealed extensive skeletal lytic lesions. Further assessment of monoclonal gammopathy revealed total protein: 10.4 g/L (normal range, 6–8), serum albumin: 1.8 g/dL (normal range, 3.5–5), and M-protein of 5.8 g/dL by serum protein electrophoresis (SPEP). Bone marrow showed sheets of plasma cells with suppressed trilineage hematopoiesis ([Fig. 1]). Flow cytometry revealed 73%. clonal plasma cells with lambda light chain restriction. Serum immunoglobulins showed elevated IgA 41 g/L (normal range, 1.03–5.91), IgG 4.91 g/L (normal range, 6.6–16.9), and IgM < 0.19 g/L (normal range, 0.37–2.58). Serum immunofixation (IFE) confirmed the presence of IgAλ band; serum-free light chains (sFLC):λ-35.1mg/L (normal range, 5.71–26.3), κ-2.20 mg/L (normal range, 3.3–19.4), and κ/λ ratio of 0.063. MALDI-TOF mass spectrometry revealed a λ monoclonal peak ([Fig. 2]). Fluorescence in situ hybridization was positive for IgH translocation and IgH-FGFR3 fusion, β2 microglobulin was 9.9 mg/L, and serum LDH was 1163 U/L (normal range, 200–400). The investigations confirmed multiple myeloma R-ISS stage III. The nerve conduction study revealed mixed axonal and demyelinating motor-sensory neuropathy of the distal nerves in the lower limb—common peroneal and sural nerves. The patient was immediately started on CyBorD chemotherapy regimen—bortezomib, cyclophosphamide, and dexamethasone. Intravenous dexamethasone was initiated at 40 mg daily for 4 days. The patient had hyperbilirubinemia—6 mg/dL (normal range, 0.2–1.3) with predominant indirect bilirubin elevation of 4.2 mg/dL and normal liver enzymes. Imaging of the liver was within normal limits. Further workup for hemolysis was negative: reticulocyte index 0.45%. (normal range, 0.5–2.5) and normal haptoglobin: 40 mg/dL (normal range, 30–200). The indirect Coombs test (ICT) and direct Coombs test (DCT) were strongly positive. The patient had symptomatic anemia for which PRC transfusion was requested. Initial testing of the patient's sample showed blood grouping discrepancy with DCT, ICT, and autocontrol positive. Because the blood bank team could not resolve the blood group, one unit of O-negative best compatible packed red cell unit was issued for emergency use. Subsequently, a B-positive blood group was confirmed; however, the presence of autoantibodies or an alloantibody could not be ruled out. Therefore, two additional units of least incompatible B-positive packed red cell units were transfused, following which the patient had worsening pulmonary congestion and altered sensorium. CSF analysis was not done as the patient had thrombocytopenia with bleeding diathesis–skin petechiae and hematuria. As there was no improvement in the patient's sensorium after initiating anti-myeloma therapy, the patient underwent plasmapheresis. Her clinical status dramatically improved, and she was continued on anti-myeloma therapy. The patient's sensorium, renal dysfunction, hypercalcemia, and thrombocytopenia gradually normalized. However, the symptomatic anemia continued to persist, requiring PRC transfusions. Hence, the patient was worked up for auto- and allo-incompatibility. Further antibody study was performed that revealed the following:
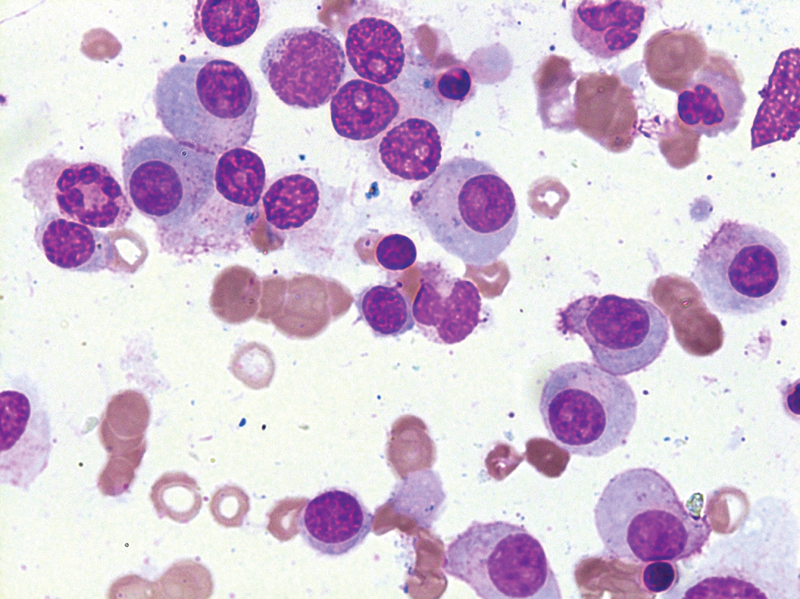
Fig 1: Leishman stain of bone marrow aspirate at 100x magnification showing atypical plasma cells.
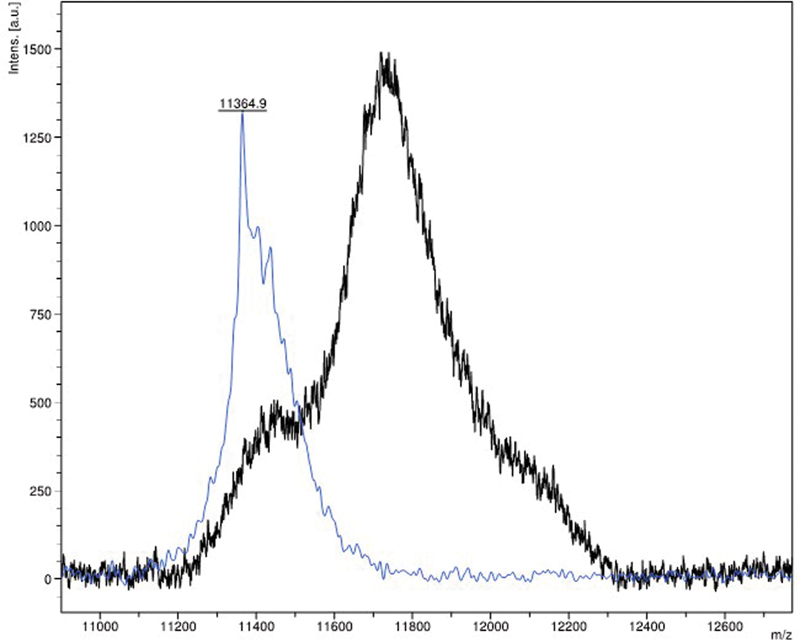
Fig 2 : MALDI-TOF mass spectrometry shows a monoclonal spike in the lambda region (blue) against a normal control (black).
Direct antiglobulin test on patient's red cells: weakly positive
Atypical antibody in patient's serum using antibody screening cells:
by saline technique (IgM phase): negative
indirect antiglobulin technique (IgG phase): positive
Patient's serum showed the presence of anti-Jka antibody: a warm IgG antibody
Presence of cold antibody
Subsequently, the patient received Jka antigen-negative B-positive compatible packed red cell unit transfusion. After that, there was no further hemolysis or drop in hemoglobin. Transfusion with compatible blood products and continuation of anti-myeloma therapy gradually normalized the hemoglobin. Cold antibody was detected; however, there were no clinical features to suggest cold agglutinin disease. The patient was subsequently started on bortezomib, lenalidomide, and dexamethasone (VRd regimen). After four cycles, the patient achieved a very good partial response with normalization of hemoglobin (Hb, 12.2 g/dL) and platelet count. The ICT continued to remain positive. The patient underwent high-dose therapy with melphalan (200 mg/m2) followed by autologous hematopoietic stem cell transplantation (ASCT). The transplant and peritransplant periods were uneventful. On subsequent follow-up, SPEP, serum IFE, sFLC, and bone marrow performed on D + 100 were suggestive of stringent complete response (sCR) and an undetectable MRD at 10−6. The patient was started on bortezomib maintenance.
Discussion
IgA type has an overall poor prognosis partly due to the frequent occurrence of high-risk cytogenetic abnormalities.[4] Anemia is one of the most common presenting features that can significantly impact physical functioning and quality of life variables.[5] Several factors can cause anemia in patients with MM, including abnormal iron utilization, inappropriately low serum erythropoietin levels, a reduction in the bone marrow response to erythropoietin, hemolysis, and bone-marrow involvement.[6] Autoimmune hemolytic anemia and cold agglutination disease are rare presenting manifestations of MM.[7] [8] Despite several reports showing the association between autoimmune hemolytic anemia and MM, none have shown conclusive evidence that the myeloma monoclonal protein is responsible, and rarely are they symptomatic due to the same.[9] There was evidence of transfusion-associated alloimmunization in our patient, which was confirmed by the identification of the anti-JKa antibody. Alloimmunization is usually a major concern among patients receiving transfusions over a prolonged duration, which is quite rare with MM.[10] Females who have received multiple transfusions are at a higher risk.[11] The frequency of hemolytic anemia due to alloimmunization is reported in 2 to 6%, of which anti-Jka antibody is the most commonly detected.[12] The reported alloimmunization rate among patients with a hemato-oncological diagnosis was 3.2%. Jka antigen belongs to the Kidd antigen blood group system. The incidence of anti-JKa antibodies in population-based studies varies from 1.3 to 2.5%.[13] In India, routine antibody screening is not done in clinical practice except in a few centers.[14] In patients who have hemolytic anemia due to alloimmunization, screening and antigen matching are recommended to prevent further complications.[15] There are instances of alloimmunization which have occurred in the immediate post-transplant period leading to hemolysis and complicating the management due to the increased transfusion requirement post-ASCT.[16] [17] Cold antibody-mediated hemolysis is usually IgM, and RBC hemolysis occurs at 3 to 4°C.[18] Therefore, cold agglutinin disease usually presents with Raynaud's phenomenon and acrocyanosis. The anti-JKa antibody-associated hemolysis is IgG-mediated. Anti-Jka antibodies are usually IgG1 and IgG3; however, some are partly IgG2, IgG4, or IgM.[13] [19] They can cause severe acute hemolytic transfusion reactions. However, more commonly, they present with delayed hemolytic transfusion reactions even up to 1 week after blood transfusions. Anti-Jka antibody is classically evanescent and difficult to detect because the levels rapidly decline in the plasma.[20] [21] Symptomatic hyperviscosity is much more common with Waldenström's macroglobulinemia (10–30%) than it is in MM (2–6%). Symptoms of hyperviscosity include mucosal bleeding, ocular neurological, and cardiovascular manifestations. Hyperviscosity is observed in IgA and IgM type paraproteinemia partly due to their higher molecular weights[22] [23]. Immediate treatment is indicated in the presence of hyperviscosity-related symptoms. Plasmapheresis immensely aids in reducing hyperviscosity-related symptoms within 1 to 2 days. Simultaneous anti-myeloma therapy with newer daratumumab-based regimens can hasten the recovery.[24] [25] Despite its aggressive disease biology, IgA MM demonstrates a good response to bortezomib-based regimens.[26] The use of anti-CD38 monoclonal antibodies, viz., daratumumab and isatuximab have implications in immunohematology; panagglutination caused by these anti-CD38 monoclonal antibodies during indirect antiglobulin testing can mask a clinically significant RBC alloantibody.[27] [28] In the present era where daratumumab is approved for use in the frontline setting in MM, RBC phenotyping or genotyping before daratumumab is recommended. This may prevent immune hemolysis and therefore ensure appropriate transfusion.[27] [28] In our patient, the identification of the anti-JKa antibody and subsequent transfusion of Jka-negative PRCs led to improvement and stabilization of hemoglobin. There was no further drop in hemoglobin, indicating that the presence of cold antibody was a silent bystander,[29] despite requiring multiple transfusions during diagnosis and peri-transplant period.
Conclusion
To our knowledge, there is no literature on patients with multiple myeloma presenting with anti-Jka antibody and a cold antibody. This case highlights the value of respecting the fine balance one needs to maintain between Occam's razor and Hickam's dictum, especially in plasma cell dyscrasias and their systemic associations.
Conflict of Interest
“Rapid and Accurate Detection of M-Protein by MALDI-TOF by Reagent-Based Extraction” Gopal Gopisetty, Nikita Mehra, Subramani Jayavelu Indian provisional patent application no: 202041009443, filed on March 5, 2020.
Acknowledgments
We thank the patient for permitting us to share all her clinical details. We thank Mr. Jayavelu for the technical support for MALDI-TOF MS M-protein analysis. We thank Dr. Anup Devasia for providing his clinical input. We would like to thank the following Institutes: Department of transfusion Medicine-RELA Institute of Medical Science, Apollo Hospitals, Chennai, and ICMR-National Institute of Immunohematology, Mumbai, for performing the detailed antibody screening.
References
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011; 364 (11) 1046-1060
- Kumar SK, Rajkumar V, Kyle RA. et al. Multiple myeloma. Nat Rev Dis Primers 2017; 3: 17046
- Kuo HH, Shen EP. Hyperviscosity retinopathy as the initial presentation of aggressive multiple myeloma. Tzu-Chi Med J 2020; 32 (04) 401-403
- Kumar SK, Rajkumar SV. The multiple myelomas - current concepts in cytogenetic classification and therapy. Nat Rev Clin Oncol 2018; 15 (07) 409-421
- Liu L, Yu Z, Cheng H. et al. Multiple myeloma hinders erythropoiesis and causes anaemia owing to high levels of CCL3 in the bone marrow microenvironment. Sci Rep 2020; 10 (01) 20508
- Birgegård G, Gascón P, Ludwig H. Evaluation of anaemia in patients with multiple myeloma and lymphoma: findings of the European CANCER ANAEMIA SURVEY. Eur J Haematol 2006; 77 (05) 378-386
- Naithani R, Dayal N. Autoimmune hemolytic anemia as presenting manifestation of multiple myeloma. Indian J Hematol Blood Transfus 2020; 36 (03) 578-579
- Pacca RL, Silva JBCBD, Souza KCE, Carbinatto RB. Autoimmune hemolytic anemia and hyperglobulinemia leading to the diagnosis of multiple myeloma. Rev Bras Hematol Hemoter 2017; 39 (04) 357-359
- Wada H, Yata K, Mikami M. et al. Multiple myeloma complicated by autoimmune hemolytic anemia. Intern Med 2004; 43 (07) 595-598
- Sanz C, Nomdedeu M, Belkaid M, Martinez I, Nomdedeu B, Pereira A. Red blood cell alloimmunization in transfused patients with myelodysplastic syndrome or chronic myelomonocytic leukemia. Transfusion 2013; 53 (04) 710-715
- Franchini M, Forni GL, Marano G. et al. Red blood cell alloimmunisation in transfusion-dependent thalassaemia: a systematic review. Blood Transfus 2019; 17 (01) 4-15
- Heddle NM, Soutar RL, O'Hoski PL. et al. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol 1995; 91 (04) 1000-1005
- Mallhi RS, Philip J, Chatterjee T, Dimri U. Presence of atypical antibody (anti Jk(a)) in a multi transfused transfusion dependent anemia patient. Med J Armed Forces India 2015; 71 (0, suppl 2) S482-S485
- Mangwana S, Kacker ANS. Red cell alloimmunization in multi-transfused, oncology patients: risks and management. Glob J Transfus Med 2019; 4 (01) 74-78
- Hauck-Dlimi B, Strobel J, Eckstein R, Zimmermann R. Prevention and management of transfusion-induced alloimmunization: current perspectives. Int J Clin Transfus Med 2014; 2: 59-63
- Young PP, Goodnough LT, Westervelt P, Diersio JF. Immune hemolysis involving non-ABO/RhD alloantibodies following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001; 27 (12) 1305-1310
- Kim MY, Chaudhary P, Shulman IA, Pullarkat V. Major non-ABO incompatibility caused by anti-Jk(a) in a patient before allogeneic hematopoietic stem cell transplantation. Immunohematology 2013; 29 (01) 11-14
- Berentsen S, Barcellini W, D'Sa S. et al. Cold agglutinin disease revisited: a multinational, observational study of 232 patients. Blood 2020; 136 (04) 480-488
- Mark K. . Fung, Brenda J. Grossman, Christopher D. Hillyer, Connie M. Westhoff JRS. Other Blood Group Systems and Antigens AABB Technical Manual; 2014
- Daniels G. Other blood groups. Tech Manual 16th ed. Bethesda, MD: AABB Press USA; 2008. ;411–436
- Sanford KW, Bourikian S, McClain A, Curtis K. Development and detection of kidd antibodies. Lab Med 2015; 46 (03) 235-240
- Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost 2003; 29 (05) 467-471
- Alkner U, Hansson UB, Lindström FD. Factors affecting IgA related hyperviscosity. Clin Exp Immunol 1983; 51 (03) 617-623
- Mina R, Bonello F, Gay F, Zamagni E, Boccadoro M. Daratumumab-based therapy for IgM multiple myeloma with hyperviscosity syndrome: a case report. Clin Lymphoma Myeloma Leuk 2021; 21 (01) e21-e24
- Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018; 132 (13) 1379-1385
- Wang L, Jin FY, Li Y. et al. IgA type multiple myeloma, clinical features, and prognosis. Chin Med J (Engl) 2018; 131 (10) 1249-1250
- Werle E, Ziebart J, Wasmund E, Eske-Pogodda K. Daratumumab interference in pretransfusion testing is overcome by addition of daratumumab Fab fragments to patients' plasma. Transfus Med Hemother 2019; 46 (06) 423-430
- Quach H, Benson S, Haysom H. et al. Considerations for pre-transfusion immunohaematology testing in patients receiving the anti-CD38 monoclonal antibody daratumumab for the treatment of multiple myeloma. Intern Med J 2018; 48 (02) 210-220
- Sefland Ø, Randen U, Berentsen S. Development of multiple myeloma of the IgA type in a patient with cold agglutinin disease: transformation or coincidence?. Case Rep Hematol 2019; 2019: 1610632
Address for correspondence
Publication History
Article published online:
28 November 2022
© 2022. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Malignancy Associated Microangiopathic Hemolytic Anemia and ThrombocytopeniaMansoor C Abdulla, Indian Journal of Medical and Paediatric Oncology, 2018
- Recurrent Infections in a Patient with Multiple MyelomaSanjay Kini, Journal of Health and Allied Sciences NU, 2019
- Hyperammonemia without liver disease as a differential diagnostic problem. Two cases with myelomaA. Horváth, Zeitschrift für Gastroenterologie, 2008
- A Case of Plasma Cell Myeloma with Multilobated and Monocytoid MorphologySneha Kakoty, Asian Journal of Oncology
- ACQUIRED FACTOR X DEFICIENCY IN MULTIPLE MYELOMA:A COMPLETE RESPONSE TO CHEMOTHERAPY.M Kos, Revista Urología Colombiana / Colombian Urology Journal, 1987
- Multiple Myeloma and Multiple Neoplasms<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Abnormal (Marker) Chromosomes in Two Patients With Acute Myelofibrosis<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Diagnosis and Management of Multiple Myeloma: A Review<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Diagnosis and Treatment of Multiple Myeloma<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Clinical Classification of Plasma Cell Myeloma<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">

Fig 1: (A)Cerebrospinal fluid (CSF) cytology shows scattered atypical blasts having high nucleus-to-cytoplasm'' ratio (N:C ratio) with opened-up nuclear chromatin, occasional prominent nucleoli, and scant amount of cytoplasm. (B) CSF cytology shows infiltration by blasts with high N:C ratio, inconspicuous nuclei, and scant amount of pale basophilic granular cytoplasm.

Fig 2 : MALDI-TOF mass spectrometry shows a monoclonal spike in the lambda region (blue) against a normal control (black).
References
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011; 364 (11) 1046-1060
- Kumar SK, Rajkumar V, Kyle RA. et al. Multiple myeloma. Nat Rev Dis Primers 2017; 3: 17046
- Kuo HH, Shen EP. Hyperviscosity retinopathy as the initial presentation of aggressive multiple myeloma. Tzu-Chi Med J 2020; 32 (04) 401-403
- Kumar SK, Rajkumar SV. The multiple myelomas - current concepts in cytogenetic classification and therapy. Nat Rev Clin Oncol 2018; 15 (07) 409-421
- Liu L, Yu Z, Cheng H. et al. Multiple myeloma hinders erythropoiesis and causes anaemia owing to high levels of CCL3 in the bone marrow microenvironment. Sci Rep 2020; 10 (01) 20508
- Birgegård G, Gascón P, Ludwig H. Evaluation of anaemia in patients with multiple myeloma and lymphoma: findings of the European CANCER ANAEMIA SURVEY. Eur J Haematol 2006; 77 (05) 378-386
- Naithani R, Dayal N. Autoimmune hemolytic anemia as presenting manifestation of multiple myeloma. Indian J Hematol Blood Transfus 2020; 36 (03) 578-579
- Pacca RL, Silva JBCBD, Souza KCE, Carbinatto RB. Autoimmune hemolytic anemia and hyperglobulinemia leading to the diagnosis of multiple myeloma. Rev Bras Hematol Hemoter 2017; 39 (04) 357-359
- Wada H, Yata K, Mikami M. et al. Multiple myeloma complicated by autoimmune hemolytic anemia. Intern Med 2004; 43 (07) 595-598
- Sanz C, Nomdedeu M, Belkaid M, Martinez I, Nomdedeu B, Pereira A. Red blood cell alloimmunization in transfused patients with myelodysplastic syndrome or chronic myelomonocytic leukemia. Transfusion 2013; 53 (04) 710-715
- Franchini M, Forni GL, Marano G. et al. Red blood cell alloimmunisation in transfusion-dependent thalassaemia: a systematic review. Blood Transfus 2019; 17 (01) 4-15
- Heddle NM, Soutar RL, O'Hoski PL. et al. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol 1995; 91 (04) 1000-1005
- Mallhi RS, Philip J, Chatterjee T, Dimri U. Presence of atypical antibody (anti Jk(a)) in a multi transfused transfusion dependent anemia patient. Med J Armed Forces India 2015; 71 (0, suppl 2) S482-S485
- Mangwana S, Kacker ANS. Red cell alloimmunization in multi-transfused, oncology patients: risks and management. Glob J Transfus Med 2019; 4 (01) 74-78
- Hauck-Dlimi B, Strobel J, Eckstein R, Zimmermann R. Prevention and management of transfusion-induced alloimmunization: current perspectives. Int J Clin Transfus Med 2014; 2: 59-63
- Young PP, Goodnough LT, Westervelt P, Diersio JF. Immune hemolysis involving non-ABO/RhD alloantibodies following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001; 27 (12) 1305-1310
- Kim MY, Chaudhary P, Shulman IA, Pullarkat V. Major non-ABO incompatibility caused by anti-Jk(a) in a patient before allogeneic hematopoietic stem cell transplantation. Immunohematology 2013; 29 (01) 11-14
- Berentsen S, Barcellini W, D'Sa S. et al. Cold agglutinin disease revisited: a multinational, observational study of 232 patients. Blood 2020; 136 (04) 480-488
- Mark K. . Fung, Brenda J. Grossman, Christopher D. Hillyer, Connie M. Westhoff JRS. Other Blood Group Systems and Antigens AABB Technical Manual; 2014
- Daniels G. Other blood groups. Tech Manual 16th ed. Bethesda, MD: AABB Press USA; 2008. ;411–436
- Sanford KW, Bourikian S, McClain A, Curtis K. Development and detection of kidd antibodies. Lab Med 2015; 46 (03) 235-240
- Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost 2003; 29 (05) 467-471
- Alkner U, Hansson UB, Lindström FD. Factors affecting IgA related hyperviscosity. Clin Exp Immunol 1983; 51 (03) 617-623
- Mina R, Bonello F, Gay F, Zamagni E, Boccadoro M. Daratumumab-based therapy for IgM multiple myeloma with hyperviscosity syndrome: a case report. Clin Lymphoma Myeloma Leuk 2021; 21 (01) e21-e24
- Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018; 132 (13) 1379-1385
- Wang L, Jin FY, Li Y. et al. IgA type multiple myeloma, clinical features, and prognosis. Chin Med J (Engl) 2018; 131 (10) 1249-1250
- Werle E, Ziebart J, Wasmund E, Eske-Pogodda K. Daratumumab interference in pretransfusion testing is overcome by addition of daratumumab Fab fragments to patients' plasma. Transfus Med Hemother 2019; 46 (06) 423-430
- Quach H, Benson S, Haysom H. et al. Considerations for pre-transfusion immunohaematology testing in patients receiving the anti-CD38 monoclonal antibody daratumumab for the treatment of multiple myeloma. Intern Med J 2018; 48 (02) 210-220
- Sefland Ø, Randen U, Berentsen S. Development of multiple myeloma of the IgA type in a patient with cold agglutinin disease: transformation or coincidence?. Case Rep Hematol 2019; 2019: 1610632


 PDF
PDF  Views
Views  Share
Share

