A diagnostic dilemma in a patient with lymphoma
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2013; 34(02): 114-116
DOI: DOI: 10.4103/0971-5851.116197
Abstract
We report the case of a 49-year-old man with a diagnosis of gastric diffuse large B cell non-Hodgkin′s lymphoma, treated with two lines of chemotherapy followed by radiotherapy, and presented about 3 months after completing therapy with recurrent episodes of epigastric pain, gastrointestinal (GI) bleeding. Computed tomography scan, positron emission tomography scan, and upper GI endoscopy revealed gastric wall thickening and lymphadenopathy. Biopsy and histopathology ultimately revealed Strongyloides stercoralis infection that was mimicking disease recurrence. Opportunistic parasitic infections represent one of the major challenges in the management of cancer patients.
Publication History
Article published online:
20 July 2021
© 2013. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
We report the case of a 49-year-old man with a diagnosis of gastric diffuse large B cell non-Hodgkin's lymphoma, treated with two lines of chemotherapy followed by radiotherapy, and presented about 3 months after completing therapy with recurrent episodes of epigastric pain, gastrointestinal (GI) bleeding. Computed tomography scan, positron emission tomography scan, and upper GI endoscopy revealed gastric wall thickening and lymphadenopathy. Biopsy and histopathology ultimately revealed Strongyloides stercoralis infection that was mimicking disease recurrence. Opportunistic parasitic infections represent one of the major challenges in the management of cancer patients.
INTRODUCTION
Gastrointestinal (GI) tract is the most frequently involved extranodal site in non-Hodgkin's lymphoma (NHL). 3% of patients presented with either hemetemesis or malena.[1] Individuals with defects in cellular immunity are prone to develop various types of opportunistic infections. Strongyloides stercoralis is found mainly in tropical and subtropical areas, and has been reported to infect 100 million people worldwide.[2] Most cases of chronic S. stercoralis infection are asymptomatic. Patients who have coexisting lymphoma and Strongyloides infection often have a fulminant course. The diagnosis of parasites often requires a high index of suspicion and the use of specialized techniques.
CASE REPORT
A 49-year-old man, with no significant co morbidities and no history of smoking or alcohol use, presented with upper abdominal pain and flatulence since 3 months. Upper GI scopy was suggestive of ulceroproliferative lesion in the gastric fundus and body. Lactate dehydrogenase (LDH) was elevated at 501 U/L. Gastric biopsy revealed lymphoma with LCA and CD20 positive. Thus, the patient was diagnosed with diffuse large B cell NHL (NHL – DLBCL) of the stomach stage I-EA. He received three cycles of cyclophosphamide, vincristine, epirubicin, and prednisolone (CEOP) chemotherapy. Following three cycles, upper GI endoscopy revealed an ulcerative lesion involving incisura and distal part of lesser curvature and part of antrum, indicative of progressive disease. He then received four cycles of salvage MINE (mitoxantrone, ifosfamide, and etoposide) following which he achieved complete remission. Patient declined further chemotherapy; therefore, he received consolidation radiation therapy with 45 GY/25# over 5 weeks to stomach and perigastric nodes.
The patient presented 2 months later with a 1-week history of malena. Examination was unremarkable. Stool occult blood was positive. Upper GI endoscopy revealed gastritis. Gastric biopsy showed chronic inflammation. Bone marrow aspiration and biopsy was normal. As there was no concrete evidence of recurrent disease, no further systemic therapy was given.
Patient again presented 3 months later with 15-day history of persistent dull aching epigastric pain. Examination was normal. Abdominopelvic computed tomography (CT) scan showed pyloric wall thickness of 1.1 cm, wall thickening around lesser curvature up to 6 mm, tiny sized perigastric nodes, and enlarged left para-aortic nodes in retroperitoneum as well as mesentery. Upper GI scopy showed mucosal thickening in the distal body and antrum of stomach, extending till pyloric rim, with the differential diagnoses being post-treatment changes or residual disease [Figure 1]. Stomach biopsy showed gastric mucosa with acute and chronic inflammation accompanying S. stercoralis larvae [Figure 2].
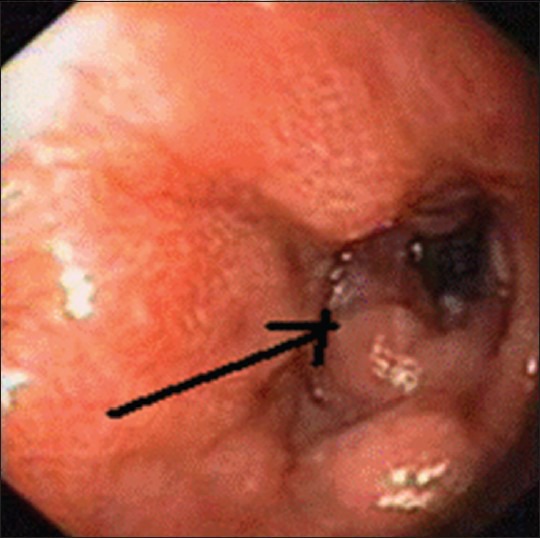
| Fig. 1 Mucosal thickening in distal body and antrum of stomach, extending till pyloric rim
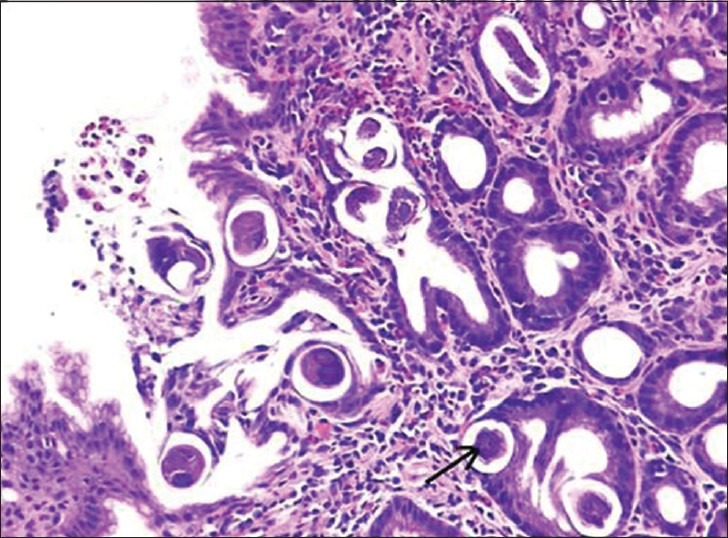
| Fig. 2 Strongyloides stercoralis larvae in the foveolar epithelium and lamina propria of gastric mucosa
The patient was treated with ivermectin 10 mg (200 g/kg) daily on two consecutive days.
After 1 month, the patient became asymptomatic. Follow-up upper GI scopy showed erosions in the distal body and antrum of stomach. Biopsy showed no organisms, parasites, or Helicobacter pylori, with no evidence of lymphoma.
The patient currently is disease free and asymptomatic over 2 years after completing treatment.
DISCUSSION
Our patient presented about 3 months after completing therapy, with recurrent episodes of epigastric pain, GI bleeding. CT scan, positron emission tomography (PET) scan, and upper GI endoscopy revealed gastric wall thickening and lymphadenopathy. Although it would be tempting to treat this constellation of symptoms and signs as recurrent disease, it would have been inappropriate in our patient. Biopsy and histopathology ultimately revealed S. stercoralis infection that was mimicking disease recurrence. This underlies the importance of a tissue diagnosis, even in the recurrent setting.
S. stercoralis is an intestinal roundworm that infects humans transcutaneously. After entering the bloodstream through the skin, the filariform larvae migrate to the lungs, ascend up the tracheobronchial tree (pulmonary migration), and descend down the GI tract to reach the small bowel.
Most cases of chronic S. stercoralis infection are asymptomatic. If present, the symptoms include GI symptoms like midepigastric pain, vomiting, either constipation or diarrhea, borborygmus, and perianal itching. Cutaneous manifestations include urticaria and rash. Rare manifestations include recurrent asthma and nephrotic syndrome. Complications of Strongyloides infection in an immunocompromised patient include bowel obstruction and GI bleeding.[3] Some people develop marked symptoms of increased larval migration, especially GI and respiratory symptoms, with a large number of parasitic larvae detectable in sputum and stool. This is called hyperinfection and is usually noted in persons with a defect in cell-mediated immunity.[4] Brain and meningeal involvement may occur in the setting of disseminated strongyloidiasis.
Patients who have coexisting lymphoma and Strongyloides infection often have a fulminant course. In a review by Genta et al. of 17 patients with disseminated Strongyloides and underlying lymphoma, the mortality rate was noted to be 82%. The causes of death included pulmonary hemorrhage causing respiratory failure or bacterial superinfection.[5] The authors suggested that this parasite should be aggressively sought out and treated prior to initiating immunosuppressive therapy. Hyperinfection of S. stercoralis has occurred in patients on doses as low as 1 mg of dexamethasone daily for 8 weeks.[6]
In our patient, the diagnosis of Strongyloides infection was delayed for an approximate period of 5 months after the onset of symptoms because of low index of suspicion. Fortunately, immunosuppressive chemotherapy and corticosteroid therapy were not administered during this period, which is probably why our patient did not develop hyperinfection syndrome, and the parasite was easily eradicated, once diagnosed.
The diagnosis of strongyloidiasis in humans is usually based on finding rhabditoid larvae in fecal material. A serological test using enzyme immunoassay is useful in immunocompetent individuals. The histologic diagnosis of strongyloidiasis from biopsies and surgical specimens is usually made incidentally in patients undergoing endoscopy for GI disease.[7]
Ivermectin is the best drug for the treatment of uncomplicated S. stercoralis infection.[8] It is well tolerated and has a higher cure rate than thiabendazole. Other drugs, such as mebendazole and albendazole, have variable therapeutic efficacy.[9]
Opportunistic parasitic infections represent one of the major challenges in the management of cancer patients. Viral and bacterial infections are readily diagnosed by well-established serologic and microbiologic methods. In contrast, the diagnosis of parasites requires a high index of suspicion and the use of specialized techniques.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Mucosal thickening in distal body and antrum of stomach, extending till pyloric rim

| Fig. 2 Strongyloides stercoralis larvae in the foveolar epithelium and lamina propria of gastric mucosa


 PDF
PDF  Views
Views  Share
Share

