A Child with Unusual Liver Lesions in a Case of Neuroblastoma
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(04): 565-566
DOI: DOI: 10.4103/ijmpo.ijmpo_205_20
A 21-month-old female initially presented to us with a 2-week history of ataxia, loss of consciousness, bruises on the forehead, nystagmus, tremor, and mydriasis with morning irritability. The findings of magnetic resonance imaging (MRI) of the abdomen were consistent with a neuroblastoma [Figure 1]. At the time of diagnosis, the tumor was localized and showed no evidence of local or regional invasion, as defined by the image-defined risk factors.
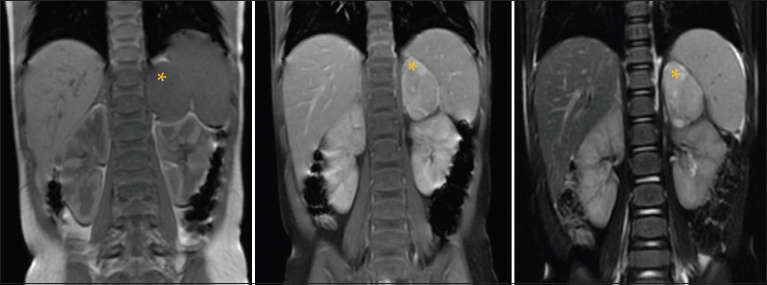
| Figure 1:Coronal T1, T1c+, and T2 images: A large left suprarenal lobulated lesion appearing hypointense on T1-weighted image with heterogeneous postcontrast enhancement. No signal voids are seen on T2-weighted image. The lesion does not cross the midline. Mass effect is seen on the adjacent spleen/kidney without invasion. There was no extension into the adjacent neural foramina. Note that the visualized liver appears normal
On surgery, the tumor was completely excised, with clear margins representing Stage 1 of the International Neuroblastoma Staging System. Histopathology was favorable with differentiating subtype and low Mitosis–Karyorrhexis index. Post surgery, MRI of the abdomen demonstrated no residual soft tissue. This was followed by intravenous immunoglobulin, prednisone, and cyclophosphamide according to standard regimen to treat the opsoclonus myoclonus ataxia syndrome. A stem cell collection was performed for autologous stem cell procedure.
At 3-month follow-up, no tumor recurrence was seen at the surgical bed. However, a single, small lesion with equivocal enhancement was discovered within the liver in segment 6/5, which was thought to be metastases versus hemangioma. Multiple follow-up MRI at 3-month interval showed gradually increasing number and size of lesions in the liver with avid enhancement but no specific washout characteristics. The biopsy of this liver lesion was attempted on two separate occasions, without a definitive diagnosis. The lesions stabilized 2 years postoperatively [Figure 2]. Still, as the possibility of metastases was thought to be highly likely, the tumor board decided to pursue open biopsy for diagnosis. The pathology on the peripheral wedge biopsy of liver surprisingly again showed no evidence of neoplasia [Figure 3]. What could be the cause of such an appearance
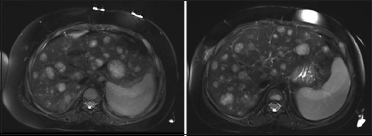
| Figure.2:Axial T2 images: Stable large liver lesions 2 years after the surgery. These lesions were found to be in keeping with extramedullary hematopoiesis
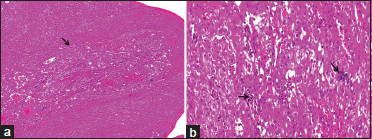
| Figure.3:Postbiopsy: (a) Low-power liver parenchyma showing a vague nodule with the arrow marking the nodule of extramedullary hematopoiesis. Contrast between dilated sinusoids in the nodule and unremarkable surrounding liver parenchyma (hematoxylin phloxine saffron stain, ×40), (b) medium-power views of the liver parenchyma showing dilated sinusoids, extramedullary hematopoiesis, and sinusoidal capillarization (hematoxylin phloxine saffron stain, ×200)
Publication History
Received: 28 April 2020
Accepted: 06 August 2020
Article published online:
17 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
A 21-month-old female initially presented to us with a 2-week history of ataxia, loss of consciousness, bruises on the forehead, nystagmus, tremor, and mydriasis with morning irritability. The findings of magnetic resonance imaging (MRI) of the abdomen were consistent with a neuroblastoma [Figure 1]. At the time of diagnosis, the tumor was localized and showed no evidence of local or regional invasion, as defined by the image-defined risk factors.

| Figure 1:Coronal T1, T1c+, and T2 images: A large left suprarenal lobulated lesion appearing hypointense on T1-weighted image with heterogeneous postcontrast enhancement. No signal voids are seen on T2-weighted image. The lesion does not cross the midline. Mass effect is seen on the adjacent spleen/kidney without invasion. There was no extension into the adjacent neural foramina. Note that the visualized liver appears normal
On surgery, the tumor was completely excised, with clear margins representing Stage 1 of the International Neuroblastoma Staging System. Histopathology was favorable with differentiating subtype and low Mitosis–Karyorrhexis index. Post surgery, MRI of the abdomen demonstrated no residual soft tissue. This was followed by intravenous immunoglobulin, prednisone, and cyclophosphamide according to standard regimen to treat the opsoclonus myoclonus ataxia syndrome. A stem cell collection was performed for autologous stem cell procedure.
At 3-month follow-up, no tumor recurrence was seen at the surgical bed. However, a single, small lesion with equivocal enhancement was discovered within the liver in segment 6/5, which was thought to be metastases versus hemangioma. Multiple follow-up MRI at 3-month interval showed gradually increasing number and size of lesions in the liver with avid enhancement but no specific washout characteristics. The biopsy of this liver lesion was attempted on two separate occasions, without a definitive diagnosis. The lesions stabilized 2 years postoperatively [Figure 2]. Still, as the possibility of metastases was thought to be highly likely, the tumor board decided to pursue open biopsy for diagnosis. The pathology on the peripheral wedge biopsy of liver surprisingly again showed no evidence of neoplasia [Figure 3]. What could be the cause of such an appearance

| Figure.2:Axial T2 images: Stable large liver lesions 2 years after the surgery. These lesions were found to be in keeping with extramedullary hematopoiesis

| Figure.3:Postbiopsy: (a) Low-power liver parenchyma showing a vague nodule with the arrow marking the nodule of extramedullary hematopoiesis. Contrast between dilated sinusoids in the nodule and unremarkable surrounding liver parenchyma (hematoxylin phloxine saffron stain, ×40), (b) medium-power views of the liver parenchyma showing dilated sinusoids, extramedullary hematopoiesis, and sinusoidal capillarization (hematoxylin phloxine saffron stain, ×200)
Answer
Neuroblastoma is a common neoplasm in the pediatric age group, usually presenting between 1 and 5 years of age. Metastases are frequent in bones and lymph nodes. Rarely, they may also be seen within the visceral organs and soft tissues.
Extramedullary hematopoiesis (EMH) is the formation of normal blood cells outside of the bone marrow, which is seen as a sequela of inadequate hematopoiesis.[1] The actual pathogenesis of EMH in patients with solid tumors remains unknown. Granulocyte colony-stimulating factor (G-CSF) is an important inducing growth factor which stimulates the bone marrow to increase the production and release of granulocytes into the blood.[2] G-CSF in turn may be an inducing factor of EMH. Our patient had received G-CSF as a stem cell rescue to ameliorate marrow suppression from chemotherapy.
A recent review described the presence of EMH in a spectrum of malignant solid tumors. They also describe liver as the most common visceral organ for EMH followed by kidney and paraspinal region.[2] However, to the best of our knowledge, the association of neuroblastoma and EMH in liver has not been reported in literature.
To conclude, in the setting of an existing malignancy, specifically neuroblastoma, EMH may pose as metastatic deposits signifying the progression of disease. In addition, childhood cancer survivors are at risk of developing pseudometastatic mass lesions, which are often multiple and develop years after completing chemotherapy. Due to prognostic implications, it is essential to confirm these lesions as they are nonaggressive with a benign course.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
References
- Kim CH. Homeostatic and pathogenic extramedullary hematopoiesis. J Blood Med 2010; 1: 13-9
- Bao Y, Liu Z, Guo M, Li B, Sun X, Wang L. Extramedullary hematopoiesis secondary to malignant solid tumors: A case report and literature review. Cancer Manag Res 2018; 10: 1461-70
Address for correspondence
Publication History
Received: 28 April 2020
Accepted: 06 August 2020
Article published
online:
17 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2,
Noida-201301 UP, India

| Figure 1:Coronal T1, T1c+, and T2 images: A large left suprarenal lobulated lesion appearing hypointense on T1-weighted image with heterogeneous postcontrast enhancement. No signal voids are seen on T2-weighted image. The lesion does not cross the midline. Mass effect is seen on the adjacent spleen/kidney without invasion. There was no extension into the adjacent neural foramina. Note that the visualized liver appears normal

| Figure.2:Axial T2 images: Stable large liver lesions 2 years after the surgery. These lesions were found to be in keeping with extramedullary hematopoiesis

| Figure.3:Postbiopsy: (a) Low-power liver parenchyma showing a vague nodule with the arrow marking the nodule of extramedullary hematopoiesis. Contrast between dilated sinusoids in the nodule and unremarkable surrounding liver parenchyma (hematoxylin phloxine saffron stain, ×40), (b) medium-power views of the liver parenchyma showing dilated sinusoids, extramedullary hematopoiesis, and sinusoidal capillarization (hematoxylin phloxine saffron stain, ×200)
References
- Kim CH. Homeostatic and pathogenic extramedullary hematopoiesis. J Blood Med 2010; 1: 13-9
- Bao Y, Liu Z, Guo M, Li B, Sun X, Wang L. Extramedullary hematopoiesis secondary to malignant solid tumors: A case report and literature review. Cancer Manag Res 2018; 10: 1461-70


 PDF
PDF  Views
Views  Share
Share

