A Case Report and Review of Literature: Epithelioid Hemangioendothelioma—An Uncommon Challenging Case
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(02): 219-225
DOI: DOI: 10.1055/s-0043-1774775
Abstract
Introduction Epithelioid hemangioendothelioma (EHE) is a rare vascular tumor of soft tissue and bone that may uncommonly occur in the liver, lung, and head and neck region. EHEs have a higher predilection for recurrence at the local site as well as distant metastasis. Surgical excision is important and is the treatment in localized diseases. A decision to give adjuvant radiotherapy should be subjective and may differ on case-to-case basis. Limited studies are available exploring the role of targeted or systemic therapy.
Case Presentation A 56-year-old lady represented with right-sided submandibular region EHE with bilateral lung metastasis. The patient underwent surgery and radiotherapy followed by targeted therapy tab pazopanib for systemic control. At 2 years of follow-up, positron emission tomography-computed tomography showed local regional control and stable systemic diseases.
Conclusion The uncertainty in choosing the most suitable treatment of EHE patients is high and may result in dissatisfactory outcomes among several patients. The present case study identified a treatment dilemma making management more challenging for rare EHE with mandibular involvement.
Keywords
hemangioendothelioma - surgery - radiotherapy - pazopanib - outcomes - case reportEthics Approval and Consent to Participate
For submission of a case report, clearance from the Institute Ethics Committee is waived at All India Institute of Medical Sciences, Jodhpur. It is notable that the patient was not subjected to any experimental investigation or treatment at any point of time.
Patient's Consent
Written informed consent was obtained from the patient's guardian for publication of this case report and accompanying images.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Authors' Contributions
S.S., B.D.: Conception and design of this study, acquisition of data, analysis and interpretation of data, and drafting. All authors read and approved the final manuscript
Publication History
Article published online:
22 September 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Pulmonary Epithelioid Hemangioendothelioma with PlGF Expression: Report of a CaseT. Haruki, The Thoracic and Cardiovascular Surgeon, 2011
- Pulmonary Epithelioid Hemangioendothelioma with PlGF Expression: Report of a CaseT. Haruki, Thorac Cardiovasc Surg, 2011
- Uncommon Cause of Left Ventricular Pseudoaneurysm: Case Report and Review of LiteratureThe Thoracic and Cardiovascular Surgeon, 2013
- Uncommon Cause of Left Ventricular Pseudoaneurysm: Case Report and Review of LiteratureThorac Cardiovasc Surg, 2013
- Epithelioid Hemangioendothelioma of Cavernous SinusRaj S. Chandran, Indian Journal of Neurosurgery, 2017
- Pseudoretinitis pigmentosa due to syphilis: a case report and literature review<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Stiff-Person Syndrome: A Case Report and Review of the Literature<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- First Case Report of FOXN1 Haploinsufficiency in China and Literature Review<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Uncommon progression of toxoplasmic papillitis: patient perception and case report<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Pseudo-strabismus secondary to macular heterotropia: a case report and literature review<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
Abstract
Introduction Epithelioid hemangioendothelioma (EHE) is a rare vascular tumor of soft tissue and bone that may uncommonly occur in the liver, lung, and head and neck region. EHEs have a higher predilection for recurrence at the local site as well as distant metastasis. Surgical excision is important and is the treatment in localized diseases. A decision to give adjuvant radiotherapy should be subjective and may differ on case-to-case basis. Limited studies are available exploring the role of targeted or systemic therapy.
Case Presentation A 56-year-old lady represented with right-sided submandibular region EHE with bilateral lung metastasis. The patient underwent surgery and radiotherapy followed by targeted therapy tab pazopanib for systemic control. At 2 years of follow-up, positron emission tomography-computed tomography showed local regional control and stable systemic diseases.
Conclusion The uncertainty in choosing the most suitable treatment of EHE patients is high and may result in dissatisfactory outcomes among several patients. The present case study identified a treatment dilemma making management more challenging for rare EHE with mandibular involvement.
Keywords
hemangioendothelioma - surgery - radiotherapy - pazopanib - outcomes - case reportIntroduction
Sarcomas are malignant tumors of the skeletal and extraskeletal connective tissue that can arise from mesenchymal tissue at any body site. Uncommon subtypes of sarcoma together account for 5%. of sarcoma tumours.[1] They are especially challenging to diagnose and treat. Epithelioid hemangioendothelioma (EHE) is an uncommon vascular sarcoma that accounts for less than 1%. of all vascular tumors.[2] The World Health Organization describes malignant EHE as an intermediate malignant neoplasm.[2] The risk of recurrence at the local site and failures distantly are high with EHEs; however, tumor-specific mortality rates may rely on their anatomic site of origin. There are only limited cases reported of EHE in the face–neck region, with an appearance in the oral cavity being extremely rare.[3] The frequently reported oral cavity site for EHE is the tongue, which accounts for 26%. of cases. This is followed by the mandibular and maxillary gingiva involvement, contributing to 22 and 19%. of cases, respectively.[4] [5] The present study describes a rare case of mandibular EHE, its management, and a summary of the literature available.
Case Presentation
A 56-year-old lady, with no comorbidities and no habits presented with right submandibular swelling which gradually increased over 2 years. On clinical examination, the submandibular mass was lobulated and soft in consistency measuring 3 × 4 cm and not fixed to the overlying skin. There were no signs of inflammation and the swelling was not tender to touch. There was a palpable left level Ib neck node of 1.5 × 2 cm in size in the contralateral neck. The rest of the head and neck area examination was noncontributory. Computed tomography (CT) imaging ([Fig. 1A]) done was suggestive of a 28 × 27 × 39 mm sized ill-defined soft tissue lesion seen in the hemimandible along the right lower alveolus which was causing destructive cortical erosion. The adjacent enhancing soft tissue component showed loss of fat planes with the right submandibular gland inferiorly. A peripherally enhancing centrally necrotic left submandibular lymphadenopathy was present measuring 18 × 17 mm in size. Whole-body positron emission tomography-CT (PET-CT) scan done was suggestive of 3 × 2.4 cm sized fluorodeoxyglucose (FDG) avid spiculated lesion in the right submandibular region, eroding overlying angle and adjacent posterior body of the right hemimandible and infiltrating the right anterior belly of the digastric and closely abutting platysma. A 1.7 × 2.2 cm sized FDG avid left cervical level Ib lymph node was also noted. Heterogeneously, FDG avid discrete cervical level Ia and bilateral level II lymph nodes were also present. Mild FDG avid multiple subpleural and parenchymal nodules were noted in bilateral lung fields suggestive of metastasis ([Fig. 1C]). Biopsy from the right submandibular region was suggestive of EHE. In view of rare disease and asymptomatic suspicious lung metastasis, the case was discussed in a multidisciplinary tumor board following which the patient was taken up for wide local excision with right segmental mandibulectomy with bilateral supraomohyoid neck dissection. Histopathology of the resected specimen was suggestive of EHE involving the right submandibular gland and underlying bone ([Fig. 2A]) with maximum tumor size of 2.8 cm, medial and lateral soft tissue margins were involved by the tumor, perineural invasion was present ([Fig. 2B]), and lymphovascular invasion was not seen. Federation Nationale des Centres de Lutte Contre Le Cancer (FNCLCC) grade 4, mitotic rate was < 1/50 high-power field (HPF), and necrosis 10%. ([Fig. 2C]). One lymph node in the left level Ib was positive (out of 5), while the right-sided nodes were negative. Tumor cells were immunoreactive for CD34 ([Fig. 2D]). In view of positive margin and lymph node involvement, the patient was planned for adjuvant radiation to a dose of 60 Gy in 30 fractions to postoperative bed and involved the lymph node region by image-guided external beam radiotherapy (RT) technique. In phase 1, 50 Gy in 25 fractions was delivered to the tumor bed and bilateral neck region, and in phase 2 (boost), 10 Gy in 5 fractions was delivered to the tumor bed and involved the nodal region, Therefore, a total of 60 Gy in 30 fractions, 2 Gy per fraction, one fraction daily, over 6 weeks along with cisplatin chemotherapy (40 mg/m2) concurrently was given once a week, which she tolerated well with grade 1 dermatitis and grade 2 mucositis. The patient recovered from acute toxicities 2 weeks after completion of RT and was started on targeted therapy tablet pazopanib (dose 400 mg) twice a day to control systemic disease and reduce the risk of locoregional failure. PET-CT scan repeated after 3 months was suggestive of postoperative fibrosis of the right submandibular region with stable pulmonary metastasis. At 2-year follow-up, the patient is asymptomatic and on tablet pazopanib dose reduced to 200 mg twice a day due to oral mucositis. Latest PET-CT showed local-regional control and metabolically inactive pulmonary metastatic diseases.
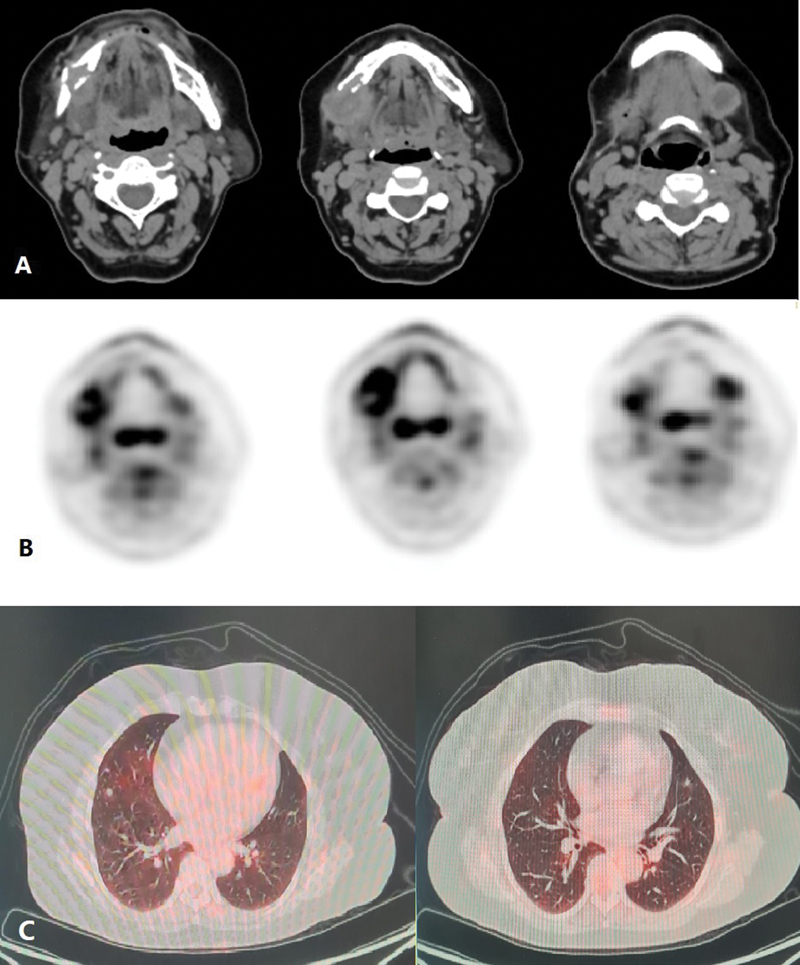
Fig 1: Contrast-enhanced computed tomography (CT) (A) and positron emission tomography (PET) images (B) showing soft tissue lesion seen in the right hemimandible causing cortical erosion and involving the submandibular gland inferiorly with an enhancing centrally necrotic left submandibular lymphadenopathy and (C) mild fluorodeoxyglucose (FDG) avid multiple parenchymal nodules were noted in bilateral lung fields suggestive of metastasis.
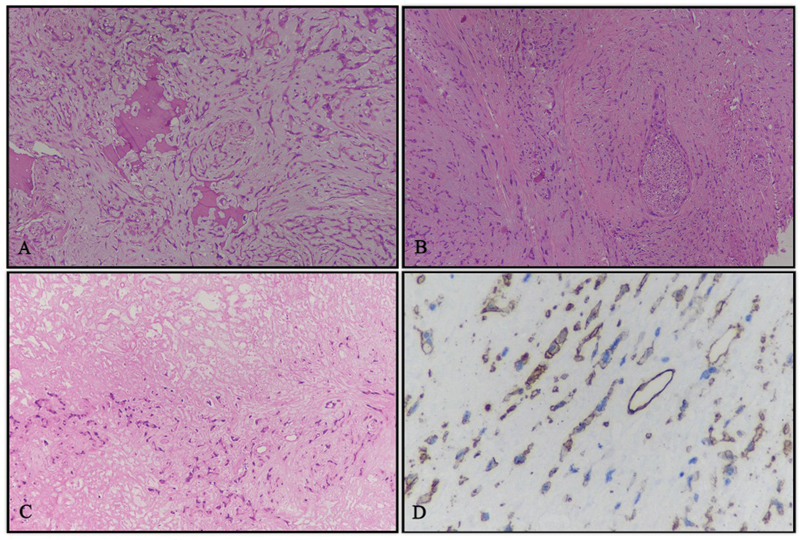
Fig 2 : Histopathological findings. (A) Cords of cells infiltrating and destroying bone and embedded in a myxohyaline matrix, hematoxylin and eosin (H&E) stain, 10 × . (B) Perineural invasion, H&E stain, 10 × . (C) Partly viable tumor and partly coagulative necrosis (in the upper half of image), H&E stain, 10 × . (D) Immunohistochemistry for CD34, showing positivity for tumor cells and interspersed vessels within the tumor, DAB-H, 10 × . H&E, hematoxylin and eosin stain; DAB-H, diaminobenzidine-hematoxylin.
Discussion
Hemangioendothelioma (HE) is a rare vascular neoplasm with an equivocal biological behavior, intermediate between highly malignant angiosarcoma and completely benign hemangiomas. HE involving the skin and soft tissue includes papillary, retiform, kaposiform, epithelioid, pseudomyogenic, and composite type.[6]
EHE is distinguished by epithelioid or histiocytoid cells with endothelial features, accounting for less than 1%. of all vascular tumors. In 1975, HE was reported initially by Dail and Liebow as pulmonary in origin.[7] Earlier, it was described as an bronchoalveolar cell carcinoma with vascular invasion with an aggressive behavior, hence, the name given was an intravascular bronchioloalveolar tumour.[8] [9] In 1982, the name EHE was coined by Weiss and Enzinger to define a vascular tumor of soft tissue and bone with characteristic features intermediate between hemangioma and angiosarcoma.[10] [11]
EHE has been considered to be the most aggressive among all types of HEs with a high risk of distant metastasis and mortality, accounting for 20 to 30%. and 10 to 20%. cases, respectively.[12] One of the largest series[6] [13] of EHE reported, recurrences at local site in 13%. of cases and regional-distant failure in approximately 31%. sites such as regional lymph nodes, lungs, liver, and bone. The authors concluded in a study with 49 patients of soft tissue EHE, that the risk of metastasis was greater in lesions > 3 cm and those showing ≥ 3 mitotic figures per 50 HPF.[14]
The etiology of EHE up to this time is unclear. At the molecular front, various angiogenic stimulators may act as promoters of endothelial cell proliferation.[15] A study suggests that for proliferation of EHE, monocyte chemoattractant protein-1 is needed and by stimulation the angiogenic nature of endothelial cell, it might promote lesions to proliferate.[16]
EHE is diagnosed predominately in female population, usually between the age group of 20 and 60 years.[17] The frequently reported symptom is pain. Cutaneous and soft tissue EHE often present as a painful mass and may cause thrombosis or occlusion in the affected vessel. Although in the present case, the mass was painless and nontender on palpation. In the majority of cases, EHE is multifocal or metastatic at diagnosis.
EHE cases show noticeable nuclear atypia with prominent nucleoli, focal and solid growth patterns, necrotic foci, and higher mitotic activity (> 2 mitoses per10 HPF) in approximately 10%. of cases. These characteristics are valuable diagnostic hints and also suggestive of the aggressive nature of the disease.[18] EHE has numerous morphological features that are indistinguishable from melanomas, carcinomas, and epithelioid sarcomas but the important differential diagnosis is with primary or metastatic carcinomas. CD31, CD34, ERG, and FLI-1 are endothelial differentiation markers frequently expressed in EHE.[19] [20] Less than 30%. of cases showed focal cytokeratin immunopositivity.[21]
EHE at a molecular level is represented by YAP1-TFE3 (10%) or WWTR1-CAMTA1 (90%) gene fusions.[22] [23] [24] The molecular characterization of EHE is highly recommended for diagnostic confirmation and rule out the differential diagnosis, like angiosarcoma and epithelioid hemangioma. Unlike EHE with WWTR1-CAMTA1 fusion, EHE with YAP1-TFE3 consist of epithelioid neoplastic cells with bright copious eosinophilic cytoplasm and focally unequivocal vasoformative features.[23] [24] Although, currently molecular study has no predictive or prognostic value, neither can it be utilized for treatment stratification purposes.
Surgical excision with regional nodal resection is the standard treatment for EHE. The main purpose of surgery is to ensure R0 resection, that is, complete resection of the tumor with microscopic negative margins. The expected cure rate in EHE after R0 resection is 70 to 80%.[25]
The risk of recurrence at the local site is approximately 10 to 15%. following complete surgical resection.[10] Although EHE is assumed to be a moderately radiosensitive tumor, the role of adjuvant RT is not well established. Indications of adjuvant RT can be extrapolated from the principles and management of soft tissue sarcomas (STS) of the extremity. Adjuvant RT can be considered in cases of close or positive margin to optimize the treatment outcome. Adjuvant RT is advisable to a dose of 60 Gy in patients with positive or close margins or cases where there is a higher risk of local recurrence. Local irradiation after resection of bone EHE up to 60 Gy showed no locoregional failures on 2 years' follow- up.[26] In the present case study, adjuvant RT was planned for the patient as medial and lateral inked margins were positive for tumor cells.
The role of preoperative RT is unclear, as there are no cases published so far for EHE. But for cases where positive or close surgical margins is expected following surgery, preoperative RT to a dose of 50 Gy in 25 fractions may be considered as per standard STS protocols. In cases where the disease is unresectable, definitive RT to a total dose of 60 Gy, 1.8 to 2 Gy/fraction has been recommended. However, depending on the clinical burden, distant metastasis, and symptoms, RT can also be delivered in a palliative setting.[17] Moreover, in neoadjuvant or adjuvant settings, none of the literature supports the use of systemic treatment in patients with resectable EHE.
Cytotoxic chemotherapy and tyrosine kinase inhibitors are the different options for systemic therapy. Although cytotoxic chemotherapy, such as single-agent gemcitabine, can be considered, vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors pazopanib, an antiangiogenic drug in phase III trial of STS, showed successful results.[27] Pazopanib resulted in clinical improvement and control of liver and lung metastasis for almost 8 years in a young female with EHE with distant metstatsis.[28] It was well tolerated with no major side effects compared to cytotoxic therapies. Pazopanib therapy was considered postsurgery and radiochemotherapy in the present study to target lung metastasis and reduce the risk of locoregional recurrence.
The efficacy of other targeted agents such as VEGFR inhibitors (bevacizumab, sorafenib), mammalian target of rapamycin (mTOR) inhibitors (sirolimus), and immunomodulatory drugs (lenalidomide) in the treatment of EHE is limited, and further studies are required to determine treatment strategies. However, mTOR inhibitors have been marked with the highest clinical activity, with progression-free survival (PFS) and overall survival of approximately 1 and 2 years, respectively. An even longer PFS has been reported in 10%. of patients.[24] The systemic approach is preferred treatment option for advanced, metastatic, and progressive EHE. Owing to the rarity of the disease, no standard treatment protocols for EHE exist. To assess clinical outcomes in EHE, a case-by-case treatment approach and follow-up strategies are needed ([Table 1]).
|
No. |
Study |
Year |
No. of cases |
Age |
Site |
Treatment |
Follow-up |
|---|---|---|---|---|---|---|---|
|
1. |
Wesley et al[29] |
1975 |
1 |
18 |
Mandibular gingiva |
Surgical excision |
NED, 2 years |
|
2. |
Mentzel et al[30] |
1997 |
5 |
30-65 |
Soft tissue, cheek and neck |
Surgical excision |
NED, 42–60 months |
|
3. |
Ebo et al[31] |
1986 |
1 |
NA |
Gingiva |
Surgical excision |
NED, 36 months |
|
4. |
Ellis and Kratochvil[32] |
1986 |
12 |
4-67 |
Neck, gingiva |
WLE and surgical excision |
LN metastases 2 cases Recurrence: 1 case |
|
5. |
Moran et al[33] |
1987 |
1 |
25 |
Palate |
Surgical excision |
NED, 21 months |
|
6. |
de Araújo et al[34] |
1987 |
1 |
4 |
Gingiva |
Surgical excision |
NA |
|
7. |
Marrogi et al[5] |
1991 |
2 |
36-45 |
Tongue, gingiva |
Surgical excision |
Recurrence: 1 case |
|
8. |
Flaitz et al[35] |
1995 |
1 |
7 |
Gingiva |
WLE |
NED, 48 months |
|
9. |
Kiryu et al[36] |
1996 |
1 |
46 |
Soft tissue, cheek |
Surgical excision |
NED, 36 months |
|
10. |
Orsini et al[37] |
2001 |
1 |
18 |
Buccal mucosa |
Surgical excision |
Recurrence: 9 months |
|
11. |
Chi et al[38] |
2005 |
1 |
28 |
Gingiva |
Surgical excision |
NED, 8 months |
|
12. |
Rigby et al[39] |
2006 |
1 |
34 |
Soft tissue, neck |
Surgical excision |
NED, 84 months |
|
13. |
Yoruk et al[40] |
2008 |
1 |
44 |
Submandibular region |
Surgical excision |
NED, 6 months |
|
14. |
Sun et al[41] |
2007 |
9 |
6-53 |
Tongue (n = 4), lip (n = 1), gingiva and alveoli of the maxilla/mandible(n = 2), buccal mucosa (n = 1), FOM (n = 1). |
Surgical excision |
NED, 6 months–8 years Recurrence in 3 cases |
|
15. |
Mohtasham et al[42] |
2008 |
1 |
9 |
Maxillary gingiva |
Surgical excision |
Recurrence, 1-year |
|
16. |
Gordón-Núñez et al[43] |
2010 |
1 |
17 |
Mandibular gingiva |
Surgical excision |
NED, 21 months |
|
17. |
Salgarelli et al[3] |
2016 |
1 |
32 |
Mandibular gingiva |
Surgical excision |
Node metastases after 4 years |
|
18 |
Ranjit et al[44] |
2015 |
1 |
25 |
Submandibular region |
Surgical excision |
NA |
|
19 |
Present case |
2021 |
1 |
56 |
Mandible and submandibular region with lung metastasis |
Surgical excision → chemoradiation → pazopanib |
On follow-up disease free |
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021; 71 (01) 7-33
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology 2014; Feb; 46 (02) 95-104
- Salgarelli AC, Bellini P, Maccio L, Setti G. Epithelioid hemangioendothelioma of the mandibular gingiva: a rare case of metastasis 4 years after radical excision and literature review. J Oral Maxillofac Pathol 2016; 20 (01) 137-141
- Ali S, Odell EW, Whaites E, Robinson PD, Challacombe SJ. Epithelioid haemangioendothelioma of the mandibular gingiva: case report and literature review. Int J Surg Case Rep 2015; 14: 194-198
- Marrogi AJ, Boyd D, el-Mofty S, Waldron C. Epithelioid hemangioendothelioma of the oral cavity: report of two cases and review of literature. J Oral Maxillofac Surg 1991; 49 (06) 633-638
- Weiss SW, Goldblum JR. Hemangioendothelioma: vascular tumors of intermediate malignancy. In: Strauss M. ed. Enzinger and Weiss's Soft Tissue Tumors. 4th ed.. St. Louis: Mosby; 2001: 891-915 [Pathol 1997; 21:363–374]
- Dail DH, Liebow AA. Intravascular bronchioloalveolar tumor. Am J Pathol 1975; 78: 6a-7a
- Wong DSY, Chiu TW, Wong GK. et al. Epithelioid haemangioendothelioma of the anterior skull base: what is the optimal treatment?. Hong Kong Med J 2009; 15 (04) 308-310
-
Dail DH, Liebow AA, Gmelich JT. et al. Intravascular, bronchiolar, and alveolar tumor of the lung (IVBAT). An analysis of twenty cases of a peculiar sclerosing endothelial tumor. Cancer 1983; 51 (03) 452-464 3.0.CO;2-M" data-id="CrossRef" data-target="CrossRef" target="linkout" href="https://doi.org/10.1002/1097-0142(19830201)51:3<452>3.0.CO;2-M" class="linkFunction" style="color: rgb(1, 52, 118); outline-width: 0px; outline-color: transparent !important; padding-right: 10px;">Crossref PubMed Search in Google Scholar
-
Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer 1982; 50 (05) 970-981 3.0.CO;2-Z" data-id="CrossRef" data-target="CrossRef" target="linkout" href="https://doi.org/10.1002/1097-0142(19820901)50:5<970>3.0.CO;2-Z" class="linkFunction" style="color: rgb(1, 52, 118); outline-width: 0px; outline-color: transparent !important; padding-right: 10px;">Crossref PubMed Search in Google Scholar
- Weiss SW, Ishak KG, Dail DH, Sweet DE, Enzinger FM. Epithelioid hemangioendothelioma and related lesions. Semin Diagn Pathol 1986; 3 (04) 259-287
- de Albuquerque AK, de Oliveira Romano S, Eisenberg AL. Epithelioid hemangioendothelioma: 15 years at the National Cancer Institute. Literature review. J Bras Patol Med Lab 2013; 49 (02) 119-125
- Pigadas N, Mohamid W, McDermott P. Epithelioid hemangioendothelioma of the parotid salivary gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 89 (06) 730-738
- Deyrup AT, Tighiouart M, Montag AG, Weiss SW. Epithelioid hemangioendothelioma of soft tissue: a proposal for risk stratification based on 49 cases. Am J Surg Pathol 2008; 32 (06) 924-927
- Radzikowska E, Szczepulska-Wójcik E, Chabowski M, Oniszh K, Langfort R, Roszkowski K. Pulmonary epithelioid haemangioendothelioma–interferon 2-alpha treatment–case report. Pneumonol Alergol Pol 2008; 76 (04) 281-285
- Gordillo GM, Onat D, Stockinger M. et al. A key angiogenic role of monocyte chemoattractant protein-1 in hemangioendothelioma proliferation. Am J Physiol Cell Physiol 2004; 287 (04) C866-C873
- Sardaro A, Bardoscia L, Petruzzelli MF, Portaluri M. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 2014; 8 (02) 259
- Righi A, Sbaraglia M, Gambarotti M. et al. Primary vascular tumors of bone: a monoinstitutional morphologic and molecular analysis of 427 cases with emphasis on epithelioid variants. Am J Surg Pathol 2020; 44 (09) 1192-1203
- Folpe AL, Chand EM, Goldblum JR, Weiss SW. Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol 2001; 25 (08) 1061-1066
- Rossi S, Orvieto E, Furlanetto A, Laurino L, Ninfo V, Dei Tos AP. Utility of the immunohistochemical detection of FLI-1 expression in round cell and vascular neoplasm using a monoclonal antibody. Mod Pathol 2004; 17 (05) 547-552
- Miettinen M, Fetsch JF. Distribution of keratins in normal endothelial cells and a spectrum of vascular tumors: implications in tumor diagnosis. Hum Pathol 2000; 31 (09) 1062-1067
- Antonescu CR, Le Loarer F, Mosquera JM. et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer 2013; 52 (08) 775-784
- Errani C, Zhang L, Sung YS. et al. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer 2011; 50 (08) 644-653
- Stacchiotti S, Miah AB, Frezza AM. et al. Epithelioid hemangioendothelioma, an ultra-rare cancer: a consensus paper from the community of experts. ESMO Open 2021; 6 (03) 100170
- Rosenbaum E, Jadeja B, Xu B. et al. Prognostic stratification of clinical and molecular epithelioid hemangioendothelioma subsets. Mod Pathol 2020; 33 (04) 591-602
- Gherman CD, Fodor D. Epithelioid hemangioendothelioma of the forearm with radius involvement. Case report. Diagn Pathol 2011; 6: 120
- Gaur S, Torabi A, O'Neill TJ. Activity of angiogenesis inhibitors in metastatic epithelioid hemangioendothelioma: a case report. Cancer Biol Med 2012; 9 (02) 133-136
- Bally O, Tassy L, Richioud B, Decouvelaere AV, Blay JY, Derbel O. Eight years tumor control with pazopanib for a metastatic resistant epithelioid hemangioendothelioma. Clin Sarcoma Res 2015; 5 (01) 12
- Wesley RK, Mintz SM, Wertheimer FW. Primary malignant hemangioendothelioma of the gingiva. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol 1975; 39 (01) 103-112
- Mentzel T, Beham A, Calonje E, Katenkamp D, Fletcher CD. Epithelioid hemangioendothelioma of skin and soft tissues: clinicopathologic and immunohistochemical study of 30 cases. Am J Surg Pathol 1997; 21 (04) 363-374
- Ebo CM, de Boever JA, Adriaens PA, Roels H. Hemangioendothelioma of the gingiva. Histopathologic and therapeutic considerations. J Clin Periodontol 1986; 13 (01) 11-18
- Ellis GL, Kratochvil FJ. Epithelioid hemangioendothelioma of the head and neck: a clinicopathologic report of twelve cases. Oral Surg Oral Med Oral Pathol 1986; 61: 61-68
- Moran WJ, Dobleman TJ, Bostwick DG. Epithelioid hemangioendothelioma (histiocytoid hemangioma) of the palate. Laryngoscope 1987; 97 (11) 1299-1302
- de Araújo VC, Marcucci G, Sesso A, de Araújo NS. Epithelioid hemangioendothelioma of the gingiva: case report and ultrastructural study. Oral Surg Oral Med Oral Pathol 1987; 63 (04) 472-477
- Flaitz CM, McDaniel RK, Mackay B, Kennady MC, Luna MA, Hicks MJ. Primary intraoral epithelioid hemangioendothelioma presenting in childhood: review of the literature and case report. Ultrastruct Pathol 1995; 19 (04) 275-279
- Kiryu H, Hashimoto H, Hori Y. Ossifying epithelioid hemangioendothelioma. J Cutan Pathol 1996; 23 (06) 558-561
- Orsini G, Fioroni M, Rubini C, Piattelli A. Epithelioid hemangioendothelioma of the oral cavity: report of case. J Oral Maxillofac Surg 2001; 59 (03) 334-337
- Chi AC, Weathers DR, Folpe AL, Dunlap DT, Rasenberger K, Neville BW. Epithelioid hemangioendothelioma of the oral cavity: report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 100 (06) 717-724
- Rigby MH, Taylor SM, Bullock MJ, Wright BA. Epithelioid hemangioendothelioma of the submandibular triangle. J Otolaryngol 2006; 35 (03) 194-195
- Yoruk O, Erdem H, Mutlu V, Erdogan F, Altas E, Kantarci M. Epithelioid hemangioendothelioma of the submandibular gland. Auris Nasus Larynx 2008; 35 (01) 157-159
- Sun ZJ, Zhang L, Zhang WF, Chen XM, Lai FM, Zhao YF. Epithelioid hemangioendothelioma of the oral cavity. Oral Dis 2007; 13 (02) 244-250
- Mohtasham N, Kharrazi AA, Jamshidi S, Jafarzadeh H. Epithelioid hemangioendothelioma of the oral cavity: a case report. J Oral Sci 2008; 50 (02) 219-223
- Gordón-Núñez MA, Silva M, Lopes MF, de Oliveira-Neto SF, Maia AP, Galvão HC. Intraoral epithelioid hemangioendothelioma: a case report and review of the literature. Med Oral Patol Oral Cir Bucal 2010; 15 (02) e340-e346
- Ranjit P, Madhusudan P, Suhaili D, Bickle I. Epitheloid hemangioendothelioma of the submandibular region. Philippine J Otolaryngol Head Neck Surg 2015; 30 (01) 47-50
Address for correspondence
Publication History
Article published online:
22 September 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Pulmonary Epithelioid Hemangioendothelioma with PlGF Expression: Report of a CaseT. Haruki, The Thoracic and Cardiovascular Surgeon, 2011
- Pulmonary Epithelioid Hemangioendothelioma with PlGF Expression: Report of a CaseT. Haruki, Thorac Cardiovasc Surg, 2011
- Uncommon Cause of Left Ventricular Pseudoaneurysm: Case Report and Review of LiteratureThorac Cardiovasc Surg, 2013
- Uncommon Cause of Left Ventricular Pseudoaneurysm: Case Report and Review of LiteratureThe Thoracic and Cardiovascular Surgeon, 2013
- Epithelioid Hemangioendothelioma of Cavernous SinusRaj S. Chandran, Indian Journal of Neurosurgery, 2017
- Two case reports of age-associated increased risk for fetomaternal hemorrhage and a literature review<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Writing a Literature Review<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Consumer Behaviour: a Literature Review<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Relationship Quality: a literature review and research agenda<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Undertaking a Literature Review in Marketing<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">

Fig 1: Contrast-enhanced computed tomography (CT) (A) and positron emission tomography (PET) images (B) showing soft tissue lesion seen in the right hemimandible causing cortical erosion and involving the submandibular gland inferiorly with an enhancing centrally necrotic left submandibular lymphadenopathy and (C) mild fluorodeoxyglucose (FDG) avid multiple parenchymal nodules were noted in bilateral lung fields suggestive of metastasis.

Fig 2 : Histopathological findings. (A) Cords of cells infiltrating and destroying bone and embedded in a myxohyaline matrix, hematoxylin and eosin (H&E) stain, 10 × . (B) Perineural invasion, H&E stain, 10 × . (C) Partly viable tumor and partly coagulative necrosis (in the upper half of image), H&E stain, 10 × . (D) Immunohistochemistry for CD34, showing positivity for tumor cells and interspersed vessels within the tumor, DAB-H, 10 × . H&E, hematoxylin and eosin stain; DAB-H, diaminobenzidine-hematoxylin.
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021; 71 (01) 7-33
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology 2014; Feb; 46 (02) 95-104
- Salgarelli AC, Bellini P, Maccio L, Setti G. Epithelioid hemangioendothelioma of the mandibular gingiva: a rare case of metastasis 4 years after radical excision and literature review. J Oral Maxillofac Pathol 2016; 20 (01) 137-141
- Ali S, Odell EW, Whaites E, Robinson PD, Challacombe SJ. Epithelioid haemangioendothelioma of the mandibular gingiva: case report and literature review. Int J Surg Case Rep 2015; 14: 194-198
- Marrogi AJ, Boyd D, el-Mofty S, Waldron C. Epithelioid hemangioendothelioma of the oral cavity: report of two cases and review of literature. J Oral Maxillofac Surg 1991; 49 (06) 633-638
- Weiss SW, Goldblum JR. Hemangioendothelioma: vascular tumors of intermediate malignancy. In: Strauss M. ed. Enzinger and Weiss's Soft Tissue Tumors. 4th ed.. St. Louis: Mosby; 2001: 891-915 [Pathol 1997; 21:363–374]
- Dail DH, Liebow AA. Intravascular bronchioloalveolar tumor. Am J Pathol 1975; 78: 6a-7a
- Wong DSY, Chiu TW, Wong GK. et al. Epithelioid haemangioendothelioma of the anterior skull base: what is the optimal treatment?. Hong Kong Med J 2009; 15 (04) 308-310
-
Dail DH, Liebow AA, Gmelich JT. et al. Intravascular, bronchiolar, and alveolar tumor of the lung (IVBAT). An analysis of twenty cases of a peculiar sclerosing endothelial tumor. Cancer 1983; 51 (03) 452-464 3.0.CO;2-M" data-id="CrossRef" data-target="CrossRef" target="linkout" href="https://doi.org/10.1002/1097-0142(19830201)51:3<452>3.0.CO;2-M" class="linkFunction" style="color: rgb(1, 52, 118); outline-width: 0px; outline-color: transparent !important; padding-right: 10px;">Crossref PubMed Search in Google Scholar
-
Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer 1982; 50 (05) 970-981 3.0.CO;2-Z" data-id="CrossRef" data-target="CrossRef" target="linkout" href="https://doi.org/10.1002/1097-0142(19820901)50:5<970>3.0.CO;2-Z" class="linkFunction" style="color: rgb(1, 52, 118); outline-width: 0px; outline-color: transparent !important; padding-right: 10px;">Crossref PubMed Search in Google Scholar
- Weiss SW, Ishak KG, Dail DH, Sweet DE, Enzinger FM. Epithelioid hemangioendothelioma and related lesions. Semin Diagn Pathol 1986; 3 (04) 259-287
- de Albuquerque AK, de Oliveira Romano S, Eisenberg AL. Epithelioid hemangioendothelioma: 15 years at the National Cancer Institute. Literature review. J Bras Patol Med Lab 2013; 49 (02) 119-125
- Pigadas N, Mohamid W, McDermott P. Epithelioid hemangioendothelioma of the parotid salivary gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 89 (06) 730-738
- Deyrup AT, Tighiouart M, Montag AG, Weiss SW. Epithelioid hemangioendothelioma of soft tissue: a proposal for risk stratification based on 49 cases. Am J Surg Pathol 2008; 32 (06) 924-927
- Radzikowska E, Szczepulska-Wójcik E, Chabowski M, Oniszh K, Langfort R, Roszkowski K. Pulmonary epithelioid haemangioendothelioma–interferon 2-alpha treatment–case report. Pneumonol Alergol Pol 2008; 76 (04) 281-285
- Gordillo GM, Onat D, Stockinger M. et al. A key angiogenic role of monocyte chemoattractant protein-1 in hemangioendothelioma proliferation. Am J Physiol Cell Physiol 2004; 287 (04) C866-C873
- Sardaro A, Bardoscia L, Petruzzelli MF, Portaluri M. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 2014; 8 (02) 259
- Righi A, Sbaraglia M, Gambarotti M. et al. Primary vascular tumors of bone: a monoinstitutional morphologic and molecular analysis of 427 cases with emphasis on epithelioid variants. Am J Surg Pathol 2020; 44 (09) 1192-1203
- Folpe AL, Chand EM, Goldblum JR, Weiss SW. Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol 2001; 25 (08) 1061-1066
- Rossi S, Orvieto E, Furlanetto A, Laurino L, Ninfo V, Dei Tos AP. Utility of the immunohistochemical detection of FLI-1 expression in round cell and vascular neoplasm using a monoclonal antibody. Mod Pathol 2004; 17 (05) 547-552
- Miettinen M, Fetsch JF. Distribution of keratins in normal endothelial cells and a spectrum of vascular tumors: implications in tumor diagnosis. Hum Pathol 2000; 31 (09) 1062-1067
- Antonescu CR, Le Loarer F, Mosquera JM. et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer 2013; 52 (08) 775-784
- Errani C, Zhang L, Sung YS. et al. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer 2011; 50 (08) 644-653
- Stacchiotti S, Miah AB, Frezza AM. et al. Epithelioid hemangioendothelioma, an ultra-rare cancer: a consensus paper from the community of experts. ESMO Open 2021; 6 (03) 100170
- Rosenbaum E, Jadeja B, Xu B. et al. Prognostic stratification of clinical and molecular epithelioid hemangioendothelioma subsets. Mod Pathol 2020; 33 (04) 591-602
- Gherman CD, Fodor D. Epithelioid hemangioendothelioma of the forearm with radius involvement. Case report. Diagn Pathol 2011; 6: 120
- Gaur S, Torabi A, O'Neill TJ. Activity of angiogenesis inhibitors in metastatic epithelioid hemangioendothelioma: a case report. Cancer Biol Med 2012; 9 (02) 133-136
- Bally O, Tassy L, Richioud B, Decouvelaere AV, Blay JY, Derbel O. Eight years tumor control with pazopanib for a metastatic resistant epithelioid hemangioendothelioma. Clin Sarcoma Res 2015; 5 (01) 12
- Wesley RK, Mintz SM, Wertheimer FW. Primary malignant hemangioendothelioma of the gingiva. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol 1975; 39 (01) 103-112
- Mentzel T, Beham A, Calonje E, Katenkamp D, Fletcher CD. Epithelioid hemangioendothelioma of skin and soft tissues: clinicopathologic and immunohistochemical study of 30 cases. Am J Surg Pathol 1997; 21 (04) 363-374
- Ebo CM, de Boever JA, Adriaens PA, Roels H. Hemangioendothelioma of the gingiva. Histopathologic and therapeutic considerations. J Clin Periodontol 1986; 13 (01) 11-18
- Ellis GL, Kratochvil FJ. Epithelioid hemangioendothelioma of the head and neck: a clinicopathologic report of twelve cases. Oral Surg Oral Med Oral Pathol 1986; 61: 61-68
- Moran WJ, Dobleman TJ, Bostwick DG. Epithelioid hemangioendothelioma (histiocytoid hemangioma) of the palate. Laryngoscope 1987; 97 (11) 1299-1302
- de Araújo VC, Marcucci G, Sesso A, de Araújo NS. Epithelioid hemangioendothelioma of the gingiva: case report and ultrastructural study. Oral Surg Oral Med Oral Pathol 1987; 63 (04) 472-477
- Flaitz CM, McDaniel RK, Mackay B, Kennady MC, Luna MA, Hicks MJ. Primary intraoral epithelioid hemangioendothelioma presenting in childhood: review of the literature and case report. Ultrastruct Pathol 1995; 19 (04) 275-279
- Kiryu H, Hashimoto H, Hori Y. Ossifying epithelioid hemangioendothelioma. J Cutan Pathol 1996; 23 (06) 558-561
- Orsini G, Fioroni M, Rubini C, Piattelli A. Epithelioid hemangioendothelioma of the oral cavity: report of case. J Oral Maxillofac Surg 2001; 59 (03) 334-337
- Chi AC, Weathers DR, Folpe AL, Dunlap DT, Rasenberger K, Neville BW. Epithelioid hemangioendothelioma of the oral cavity: report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 100 (06) 717-724
- Rigby MH, Taylor SM, Bullock MJ, Wright BA. Epithelioid hemangioendothelioma of the submandibular triangle. J Otolaryngol 2006; 35 (03) 194-195
- Yoruk O, Erdem H, Mutlu V, Erdogan F, Altas E, Kantarci M. Epithelioid hemangioendothelioma of the submandibular gland. Auris Nasus Larynx 2008; 35 (01) 157-159
- Sun ZJ, Zhang L, Zhang WF, Chen XM, Lai FM, Zhao YF. Epithelioid hemangioendothelioma of the oral cavity. Oral Dis 2007; 13 (02) 244-250
- Mohtasham N, Kharrazi AA, Jamshidi S, Jafarzadeh H. Epithelioid hemangioendothelioma of the oral cavity: a case report. J Oral Sci 2008; 50 (02) 219-223
- Gordón-Núñez MA, Silva M, Lopes MF, de Oliveira-Neto SF, Maia AP, Galvão HC. Intraoral epithelioid hemangioendothelioma: a case report and review of the literature. Med Oral Patol Oral Cir Bucal 2010; 15 (02) e340-e346
- Ranjit P, Madhusudan P, Suhaili D, Bickle I. Epitheloid hemangioendothelioma of the submandibular region. Philippine J Otolaryngol Head Neck Surg 2015; 30 (01) 47-50


 PDF
PDF  Views
Views  Share
Share

