5-Fluorouracil-Induced Leukoencephalopathy: Report of Two Cases and Review of Literature
CC BY 4.0 · Indian J Med Paediatr Oncol 2024; 45(06): 546-549
DOI: DOI: 10.1055/s-0044-1779488
5-fluorouracil (5FU) forms an important component of chemotherapy regimens used in various gastrointestinal (GI) adenocarcinomas and head and neck squamous cell carcinomas. Leukoencephalopathy is a rare adverse effect of 5FU, mediated by hyperammonemia and hyperlactatemia. We report cases of two patients with GI adenocarcinomas who developed neurological symptoms while on 5FU infusion. The neuroimaging and biochemical parameters were suggestive of toxic leukoencephalopathy. They were managed with cessation of the drug and short-term antiepileptic therapy. We also discuss the pathophysiology of this adverse effect and its management.
Patient Consent
None declared.
Author Contributions
S.R.N. wrote the manuscript and contributed to the intellectual content of the study. S.H. and A.J. reviewed the manuscript and contributed to the intellectual content of the study. The manuscript has been read and approved by all the authors and it represents honest work.
Publication History
Article published online:
27 September 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
We recommend
- Pituicytoma: A Report of Two Cases and Literature ReviewK Giridharan, Indian Journal of Neurosurgery
- Pancreaticopleural fistula: Report of two cases and review of literaturePiyush Ranjan, Journal of Digestive Endoscopy, 2013
- Pancreaticopleural fistula: Report of two cases and review of literaturePiyush Ranjan, Journal of Digestive Endoscopy, 2013
- Emphysematous osteomyelitis: Report of two cases and review of literatureSachin Khanduri, Indian J Radiol Imaging, 2018
- Two Cases with Progressive Cystic LeukoencephalopathyZ. Yapici, Neuropediatrics, 2009
- Pityriasis Rubra Pilaris: A Report of Two Cases and Literature Review Mirjana Paravina, Serbian Journal of Dermatology and Venereology, 2016
- Retroperitoneal cystic lymphangioma: Report of two cases and review of the literature Brij M. Rekhi, Cleveland Clinic Journal of Medicine, 1972
- Terra Firma Forme Dermatosis – a Report of two Cases and a Review of the Literature Katarina Runtić, Serbian Journal of Dermatology and Venereology, 2019
- Hyperfibrinogenemia in Peripheral Arterial Disease: Coexistent and Independent Risk Factor (A Report of Two Cases and Review of Literature) Marijan Bosevski, PRILOZI, 2018
- Spigelian hernia: A review of the literature and report of three cases Norman R. Hertzer, Cleveland Clinic Journal of Medicine, 1971
5-fluorouracil (5FU) forms an important component of chemotherapy regimens used in various gastrointestinal (GI) adenocarcinomas and head and neck squamous cell carcinomas. Leukoencephalopathy is a rare adverse effect of 5FU, mediated by hyperammonemia and hyperlactatemia. We report cases of two patients with GI adenocarcinomas who developed neurological symptoms while on 5FU infusion. The neuroimaging and biochemical parameters were suggestive of toxic leukoencephalopathy. They were managed with cessation of the drug and short-term antiepileptic therapy. We also discuss the pathophysiology of this adverse effect and its management.
Keywords
5-fluorouracil - leukoencephalopathy - GI malignancies - neurotoxicityIntroduction
Mucositis, diarrhea, and myelosuppression are the main toxicities of bolus dosing, while hand-foot syndrome and mucositis are the toxicities seen with longer infusions.[1] Acute cerebellar syndrome characterized by ataxia, slurred speech, and nystagmus is the commonest neurotoxicity seen with 5-fluorouracil (5FU). Other reported neurological complication of 5FU is cognitive impairment.[2] The incidence of 5FU-related encephalopathy was 5.7%-in a recent retrospective study.[3] With 5FU being one of the commonest drugs used in solid tumors, the odds of encountering a rare Complication like leukoencephalopathy are still high. Our case series elucidates the course of this complication in two patients and their management.
Case 1
A 41-year-old woman without any comorbidities underwent radical left hemicolectomy for adenocarcinoma of the transverse colon. Histopathology showed a pT3N1 disease. The first cycle of adjuvant chemotherapy, modified 5FU + leucovorin + oxaliplatin (mFOLFOX 6) regimen, was started. After 36 hours of infusion, she developed an episode of generalized tonic–clonic seizure. Arterial blood gas (ABG) analysis showed a lactate level of 6.8 mmol/L. Other metabolic parameters including renal and liver function tests, blood glucose, and carbon dioxide levels were normal. Diffusion-weighted (DW) sequences of magnetic resonance imaging (MRI) of the brain showed an inverted V-shaped hyperintense lesions in the splenium of the corpus callosum and bilateral centrum semiovale, with corresponding hypointensities on apparent diffusion coefficient (ADC) sequence images ([Fig. 1A]), suggestive of acute toxic leukoencephalopathy. No corresponding abnormality was found on T2-weighted (T2W) sequences ([Fig. 1B]). 5FU infusion was stopped and she was treated with levetiracetam. She recovered completely without any neurological deficit and serum lactate level was normal at the time of discharge. Dihydropyrimidine dehydrogenase (DPD) mutation was negative by polymerase chain reaction (PCR). 5FU was discontinued permanently and she did not have recurrence of symptoms on follow-up after 3 months. She completed further adjuvant chemotherapy as capecitabine plus oxaliplatin (CapOx) and continues to be disease free.
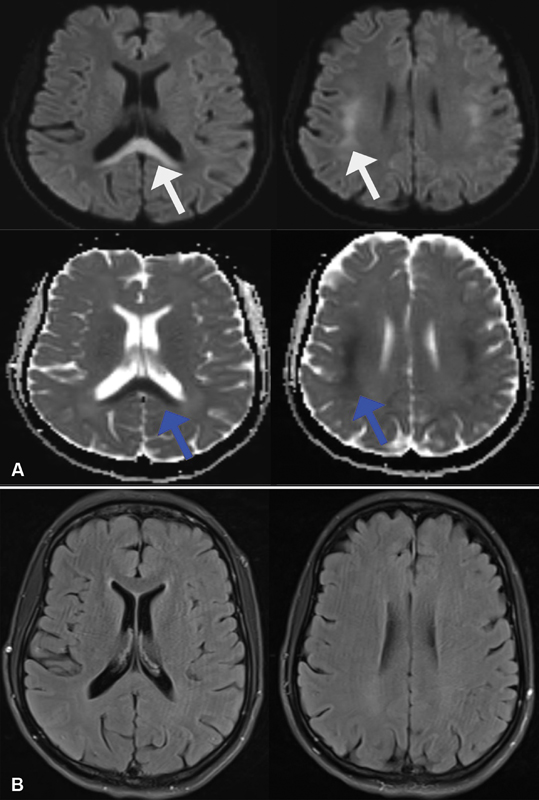
| Fig 1 (A) Diffusion-weighted (DW) sequence showing inverted hyperintensities in the corpus callosum and bilateral subcortical regions (white arrow) with corresponding hypointensities on apparent diffusion coefficient (ADC) sequences (blue arrows). (B) T2-weighted (T2W) sequences not showing any corresponding abnormality.|
Case 2
A 36-year-old woman without any comorbidities was diagnosed with metastatic adenocarcinoma of the stomach and was started on mFOLFOX 6 regimen. After 40 hours of infusion, she developed acute onset tremors in the left upper limb. Neurological examination was normal except for the tremors. 5FU infusion was stopped. Her blood lactate level was 3.8 mmol/L and ammonia was 156 mcg/dL. Other metabolic parameters were normal. MRI of the brain showed inverted V-shaped hyperintense lesions in the corpus callosum and bilateral centrum semiovale on DW images, with corresponding hypointensity on ADC and subtle hyperintensity on T2W images ([Fig. 2]). Tremors resolved spontaneously after stopping 5FU. DPD mutation test by PCR was negative. She was started on CapOx regimen subsequently, which she is tolerating well without any recurrence of neurological symptoms.
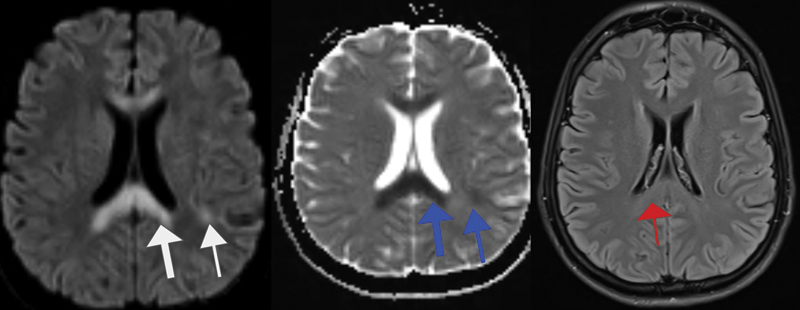
| Fig 2Diffusion-weighted (DW) sequence showing inverted hyperintensities in the corpus callosum and bilateral subcortical regions (white arrow) with corresponding hypointensities on apparent diffusion coefficient sequences (blue arrows) and subtle hyperintensity in the corpus callosum on T2-weighted (T2W) sequence (red arrow).
Discussion
The metabolism of 5FU is mediated by DPD.[4] Ammonia is the end product of 5FU metabolism. When a high dose of 5FU is administered, a proportion of 5FU is metabolized to fluoroacetate. Fluoroacetate inhibits the Krebs cycle resulting in adenosine triphosphate (ATP) deficiency. ATP-dependent urea cycle is responsible for metabolism of ammonia to an excretable form of urea. Lack of ATP results in increased blood ammonia. On the other hand, inhibition of the Krebs cycle results in increased conversion of pyruvate, an end product of glycolysis, into lactate, which is mediated by lactate dehydrogenase. Hyperammonemia and hyperlactatemia lead to leukoencephalopathy.[3]
DPD deficiency is usually associated with severe 5FU-related toxicities like mucositis, diarrhea, myelosuppression, and hand-foot syndrome.[4] Contrary to this, previous case series reported that 5FU leukoencephalopathy is unrelated to DPD deficiency.[5] This is also evident in our patients, both of whom did not have DPD deficiency. It is noteworthy that our patients did not suffer from any of the common 5FU toxicities, similar to the patients described in the retrospective series by Yeh and Cheng.[3] This shows that leukoencephalopathy is due to the end products of 5FU metabolism, rather than 5FU itself.
A wide spectrum of clinical manifestations varying from slurred speech and focal seizures to cognitive impairment and generalized tonic–clonic seizures has been reported.[3] [5] [6] [7] One of our patients had upper limb tremors as the sole manifestation. Symptoms are completely reversible once the drug is stopped.[3]
MRI of the brain with DW and ADC sequences is the imaging of choice to diagnose toxic leukoencephalopathy.[8] Hyperintensities involving the corpus callosum and deep white matter on DW and T2W sequences are the typical findings on MRI.[9] In early phases, T2W sequences might show faint hyperintensity with corresponding marked hyperintensity in DW sequences.[10] This could be the reason why T2W sequences did not show any abnormality in one of our patients.
In a case series by Jose et al, all five patients safely continued capecitabine-based chemotherapy as an alternative to 5FU.[5] Our patients also did not develop any neurological symptoms due to capecitabine. This suggests that capecitabine might be a good alternative to those who develop 5FU-induced leukoencephalopathy. However, there are reports of capecitabine-induced leukoencephalopathy as well.[11] [12] The literature showed only two case reports of leukoencephalopathy related to tegafur uracil (TFU), another derivative of 5FU.[13] [14] Switching to capecitabine or TFU can be considered carefully after discussing the risks of patients developing leukoencephalopathy due to 5FU.
One should also be aware of oxaliplatin-induced posterior reversible encephalopathy syndrome (PRES).[15] [16] However, oxaliplatin-induced encephalopathy without typical features of PRES has also been reported. This is a consequence of hyperammonemia caused by oxaliplatin.[17] Our patients received oxaliplatin even after the neurological events without experiencing the events again. This suggests that the encephalopathy in this case might not be related to oxaliplatin.
Leukoencephalopathy is also a complication of newer anticancer agents like antibody drug conjugates. Brentuximab vedotin and polatuzumab vedotin are reported to cause progressive multifocal leukoencephalopathy (PML), a fatal manifestation of reactivation of latent John Cunningham (JC) polyomavirus infection.[18] [19] Vascular endothelial growth factor receptor targeting agents like sunitinib, axitinib, lenvatinib, and pazopanib are also known to cause PRES.[20] [21] [22] [23] On the other hand, immune checkpoint inhibitors have been used to treat PML.[24]
In the era of newer anticancer drug innovations, it is equally important to promptly recognize, learn, and report the rare toxicities of conventional chemotherapeutic agents because these drugs continue to remain the backbone of anticancer therapy. However, the biggest limitation of reporting a rare toxicity is that it is often in the form of case reports/series, which do not become a part of robust data analysis to generate solid evidence.
Conclusions
5FU-induced leukoencephalopathy is a completely reversible neurological condition that needs prompt identification and timely management. It is important to check the DW and ADC sequences if T2W sequences are normal on brain MRI to diagnose correctly in the early phases of leukoencephalopathy. Capecitabine seems to be a reasonable alternative choice in these patients.
Conflict of Interest
None declared.
Patient Consent
None declared.
Author Contributions
S.R.N. wrote the manuscript and contributed to the intellectual content of the study. S.H. and A.J. reviewed the manuscript and contributed to the intellectual content of the study. The manuscript has been read and approved by all the authors and it represents honest work.
References
- Poon MA, O'Connell MJ, Moertel CG. et al. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol 1989; 7 (10) 1407-1418CrossrefPubMedSearch in Google Scholar
- Moore DH, Fowler Jr WC, Crumpler LS. 5-fluorouracil neurotoxicity. Gynecol Oncol 1990; 36 (01) 152-154CrossrefPubMedSearch in Google Scholar
- Yeh KH, Cheng AL. High-dose 5-fluorouracil infusional therapy is associated with hyperammonaemia, lactic acidosis and encephalopathy. Br J Cancer 1997; 75 (03) 464-465CrossrefPubMedSearch in Google Scholar
- Falvella FS, Caporale M, Cheli S. et al. Undetected toxicity risk in pharmacogenetic testing for dihydropyrimidine dehydrogenase. Int J Mol Sci 2015; 16 (04) 8884-8895CrossrefPubMedSearch in Google Scholar
- Jose N, Joel A, Selvakumar RJ. et al. Diagnosis and management of 5-fluorouracil (5-FU)-induced acute leukoencephalopathy: lessons learnt from a single-centre case series. J Egypt Natl Canc Inst 2022; 34 (01) 22CrossrefPubMedSearch in Google Scholar
- Akitake R, Miyamoto S, Nakamura F. et al. Early detection of 5-FU-induced acute leukoencephalopathy on diffusion-weighted MRI. Jpn J Clin Oncol 2011; 41 (01) 121-124CrossrefPubMedSearch in Google Scholar
- Ki SS, Jeong JM, Kim SH. et al. A case of neurotoxicity following 5-fluorouracil-based chemotherapy. Korean J Intern Med (Korean Assoc Intern Med) 2002; 17 (01) 73-77Search in Google Scholar
- Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med 2001; 345 (06) 425-432CrossrefPubMedSearch in Google Scholar
- Lee WW, Kim JS, Son KR, Kwon HM. Atypical diffusion-restricted lesion in 5-fluorouracil encephalopathy. AJNR Am J Neuroradiol 2012; 33 (07) E102-E103CrossrefPubMedSearch in Google Scholar
- Lucato LT, McKinney AM, Short J, Teksam M, Truwit CL. Reversible findings of restricted diffusion in 5-fluorouracil neurotoxicity. Australas Radiol 2006; 50 (04) 364-368CrossrefPubMedSearch in Google Scholar
- Coshimura K, Tokunaga S, Daga H, Inoue M. Capecitabine-induced leukoencephalopathy. Intern Med 2019; 58 (04) 621-622CrossrefPubMedSearch in Google Scholar
- Videnovic A, Semenov I, Chua-Adajar R. et al. Capecitabine-induced multifocal leukoencephalopathy: a report of five cases. Neurology 2005; 65 (11) 1792-1794 , discussion 1685CrossrefPubMedSearch in Google Scholar
- Ohara S, Hayashi R, Hata S, Itoh N, Hanyu N, Yamamoto K. Leukoencephalopathy induced by chemotherapy with tegafur, a 5-fluorouracil derivative. Acta Neuropathol 1998; 96 (05) 527-531CrossrefPubMedSearch in Google Scholar
- Hayashi R, Hanyu N, Kitahara A. Leukoencephalopathy induced by tegafur: serial studies of somatosensory evoked potentials and cerebrospinal fluid. Intern Med 1992; 31 (06) 828-831CrossrefPubMedSearch in Google Scholar
- Janjua TK, Hassan M, Afridi HK, Zahid NA. Oxaliplatin-induced posterior reversible encephalopathy syndrome (PRES). BMJ Case Rep 2017; 2017: bcr2017221571CrossrefPubMedSearch in Google Scholar
- Ogata T, Satake H, Ogata M, Hatachi Y, Yasui H. Oxaliplatin-induced hyperammonemic encephalopathy in a patient with metastatic pancreatic cancer: a case report. Case Rep Oncol 2017; 10 (03) 885-889CrossrefPubMedSearch in Google Scholar
- Rahal AK, Truong PV, Kallail KJ. Oxaliplatin-induced tonic-clonic seizures. Case Rep Oncol Med 2015; 2015: 879217PubMedSearch in Google Scholar
- Carson KR, Newsome SD, Kim EJ. et al. Progressive multifocal leukoencephalopathy associated with brentuximab vedotin therapy: a report of 5 cases from the Southern Network on Adverse Reactions (SONAR) project. Cancer 2014; 120 (16) 2464-2471CrossrefPubMedSearch in Google Scholar
- Sehn LH, Herrera AF, Flowers CR. et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2020; 38 (02) 155-165CrossrefPubMedSearch in Google Scholar
- Martín G, Bellido L, Cruz JJ. Reversible posterior leukoencephalopathy syndrome induced by sunitinib. J Clin Oncol 2007; 25 (23) 3559CrossrefPubMedSearch in Google Scholar
- Levy A, Benmoussa L, Ammari S, Albiges L, Escudier B. Reversible posterior leukoencephalopathy syndrome induced by axitinib. Clin Genitourin Cancer 2014; 12 (01) e33-e34CrossrefPubMedSearch in Google Scholar
- Cabanillas ME, Habra MA. Lenvatinib: role in thyroid cancer and other solid tumors. Cancer Treat Rev 2016; 42: 47-55CrossrefPubMedSearch in Google Scholar
- Chelis L, Souftas V, Amarantidis K. et al. Reversible posterior leukoencephalopathy syndrome induced by pazopanib. BMC Cancer 2012; 12: 489CrossrefPubMedSearch in Google Scholar
- Roos-Weil D, Weiss N, Guihot A. et al. Immune checkpoint inhibitors for progressive multifocal leukoencephalopathy: a new gold standard?. J Neurol 2021; 268 (07) 2458-2465CrossrefPubMedSearch in Google Scholar
Address for correspondence
Dr Supreeth R.N.“Pragati” 30-276/14/21 & 22, Dwarakamai colony, Old Safilguda, Shafi Nagar, Secunderabad - 500056IndiaEmail: supreeth22@gamil.comPublication History
Article published online:
27 September 2024© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, IndiaWe recommend- Pituicytoma: A Report of Two Cases and Literature ReviewK Giridharan, Indian Journal of Neurosurgery
- Pancreaticopleural fistula: Report of two cases and review of literaturePiyush Ranjan, Journal of Digestive Endoscopy, 2013
- Pancreaticopleural fistula: Report of two cases and review of literaturePiyush Ranjan, Journal of Digestive Endoscopy, 2013
- Emphysematous osteomyelitis: Report of two cases and review of literatureSachin Khanduri, Indian J Radiol Imaging, 2018
- Two Cases with Progressive Cystic LeukoencephalopathyZ. Yapici, Neuropediatrics, 2009
- Gemcitabine-Induced Reversible Posterior Leukoencephalopathy Syndrome: A Case Report and Review of the Literature Anita Rajasekhar, The Oncologist, 2007
- Idiopathic pulmonary haemosiderosis: report of two cases and review of the literature P Bailey, Postgraduate Medical Journal, 1979
- The Traumatic Bone Cyst: Review of Literature and Report of Two Cases Christopher G. Fielding, Military Medicine, 1992
- Listeria Monocytogenes Meningitis: Report of two Cases and Review of the Literature Edward S. Spilkin, American Journal of Clinical Pathology, 1968
- Pneumatosis Cystoides Intestinalis: Report of Two Cases and Review of the Literature D. Scott Finelli, Military Medicine, 1987
- Pituicytoma: A Report of Two Cases and Literature Review

| Fig 1 (A) Diffusion-weighted (DW) sequence showing inverted hyperintensities in the corpus callosum and bilateral subcortical regions (white arrow) with corresponding hypointensities on apparent diffusion coefficient (ADC) sequences (blue arrows). (B) T2-weighted (T2W) sequences not showing any corresponding abnormality.|

| Fig 2Diffusion-weighted (DW) sequence showing inverted hyperintensities in the corpus callosum and bilateral subcortical regions (white arrow) with corresponding hypointensities on apparent diffusion coefficient sequences (blue arrows) and subtle hyperintensity in the corpus callosum on T2-weighted (T2W) sequence (red arrow).
References
- Poon MA, O'Connell MJ, Moertel CG. et al. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol 1989; 7 (10) 1407-1418CrossrefPubMedSearch in Google Scholar
- Moore DH, Fowler Jr WC, Crumpler LS. 5-fluorouracil neurotoxicity. Gynecol Oncol 1990; 36 (01) 152-154CrossrefPubMedSearch in Google Scholar
- Yeh KH, Cheng AL. High-dose 5-fluorouracil infusional therapy is associated with hyperammonaemia, lactic acidosis and encephalopathy. Br J Cancer 1997; 75 (03) 464-465CrossrefPubMedSearch in Google Scholar
- Falvella FS, Caporale M, Cheli S. et al. Undetected toxicity risk in pharmacogenetic testing for dihydropyrimidine dehydrogenase. Int J Mol Sci 2015; 16 (04) 8884-8895CrossrefPubMedSearch in Google Scholar
- Jose N, Joel A, Selvakumar RJ. et al. Diagnosis and management of 5-fluorouracil (5-FU)-induced acute leukoencephalopathy: lessons learnt from a single-centre case series. J Egypt Natl Canc Inst 2022; 34 (01) 22CrossrefPubMedSearch in Google Scholar
- Akitake R, Miyamoto S, Nakamura F. et al. Early detection of 5-FU-induced acute leukoencephalopathy on diffusion-weighted MRI. Jpn J Clin Oncol 2011; 41 (01) 121-124CrossrefPubMedSearch in Google Scholar
- Ki SS, Jeong JM, Kim SH. et al. A case of neurotoxicity following 5-fluorouracil-based chemotherapy. Korean J Intern Med (Korean Assoc Intern Med) 2002; 17 (01) 73-77Search in Google Scholar
- Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med 2001; 345 (06) 425-432CrossrefPubMedSearch in Google Scholar
- Lee WW, Kim JS, Son KR, Kwon HM. Atypical diffusion-restricted lesion in 5-fluorouracil encephalopathy. AJNR Am J Neuroradiol 2012; 33 (07) E102-E103CrossrefPubMedSearch in Google Scholar
- Lucato LT, McKinney AM, Short J, Teksam M, Truwit CL. Reversible findings of restricted diffusion in 5-fluorouracil neurotoxicity. Australas Radiol 2006; 50 (04) 364-368CrossrefPubMedSearch in Google Scholar
- Yoshimura K, Tokunaga S, Daga H, Inoue M. Capecitabine-induced leukoencephalopathy. Intern Med 2019; 58 (04) 621-622CrossrefPubMedSearch in Google Scholar
- Videnovic A, Semenov I, Chua-Adajar R. et al. Capecitabine-induced multifocal leukoencephalopathy: a report of five cases. Neurology 2005; 65 (11) 1792-1794 , discussion 1685CrossrefPubMedSearch in Google Scholar
- Ohara S, Hayashi R, Hata S, Itoh N, Hanyu N, Yamamoto K. Leukoencephalopathy induced by chemotherapy with tegafur, a 5-fluorouracil derivative. Acta Neuropathol 1998; 96 (05) 527-531CrossrefPubMedSearch in Google Scholar
- Hayashi R, Hanyu N, Kitahara A. Leukoencephalopathy induced by tegafur: serial studies of somatosensory evoked potentials and cerebrospinal fluid. Intern Med 1992; 31 (06) 828-831CrossrefPubMedSearch in Google Scholar
- Janjua TK, Hassan M, Afridi HK, Zahid NA. Oxaliplatin-induced posterior reversible encephalopathy syndrome (PRES). BMJ Case Rep 2017; 2017: bcr2017221571CrossrefPubMedSearch in Google Scholar
- Ogata T, Satake H, Ogata M, Hatachi Y, Yasui H. Oxaliplatin-induced hyperammonemic encephalopathy in a patient with metastatic pancreatic cancer: a case report. Case Rep Oncol 2017; 10 (03) 885-889CrossrefPubMedSearch in Google Scholar
- Rahal AK, Truong PV, Kallail KJ. Oxaliplatin-induced tonic-clonic seizures. Case Rep Oncol Med 2015; 2015: 879217PubMedSearch in Google Scholar
- Carson KR, Newsome SD, Kim EJ. et al. Progressive multifocal leukoencephalopathy associated with brentuximab vedotin therapy: a report of 5 cases from the Southern Network on Adverse Reactions (SONAR) project. Cancer 2014; 120 (16) 2464-2471CrossrefPubMedSearch in Google Scholar
- Sehn LH, Herrera AF, Flowers CR. et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2020; 38 (02) 155-165CrossrefPubMedSearch in Google Scholar
- Martín G, Bellido L, Cruz JJ. Reversible posterior leukoencephalopathy syndrome induced by sunitinib. J Clin Oncol 2007; 25 (23) 3559CrossrefPubMedSearch in Google Scholar
- Levy A, Benmoussa L, Ammari S, Albiges L, Escudier B. Reversible posterior leukoencephalopathy syndrome induced by axitinib. Clin Genitourin Cancer 2014; 12 (01) e33-e34CrossrefPubMedSearch in Google Scholar
- Cabanillas ME, Habra MA. Lenvatinib: role in thyroid cancer and other solid tumors. Cancer Treat Rev 2016; 42: 47-55CrossrefPubMedSearch in Google Scholar
- Chelis L, Souftas V, Amarantidis K. et al. Reversible posterior leukoencephalopathy syndrome induced by pazopanib. BMC Cancer 2012; 12: 489CrossrefPubMedSearch in Google Scholar
- Roos-Weil D, Weiss N, Guihot A. et al. Immune checkpoint inhibitors for progressive multifocal leukoencephalopathy: a new gold standard?. J Neurol 2021; 268 (07) 2458-2465CrossrefPubMedSearch in Google Scholar


 PDF
PDF  Views
Views  Share
Share

