131I-mIBG Therapy in the Management of High-Risk Neuroblastoma: A Retrospective Study from a Tertiary Level Hospital in South India
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(01): 071-076
DOI: 10.1055/s-0044-1787303
Abstract
Introduction Neuroblastoma is the most common extracranial solid tumor in childhood. The data on the treatment experience with 131iodine-meta-iodo-benzyl-guanidine (131I-mIBG) and clinical outcome data are meager from India.
Objectives This article studies the efficacy and treatment outcomes in patients treated with 131I-mIBG in high-risk neuroblastoma.
Materials and Methods The study group consisted of 201 consecutive patients (aged between 1 and 15 years) with biopsy-proven neuroblastoma who underwent 131I-mIBG scans from 2012 to 2022. The majority of these children had a disease that was inoperable or had poor response to chemotherapy. Patients with positive scintigraphy were considered for therapy with 131I-mIBG. The findings were analyzed and correlated with the final diagnosis and outcomes obtained from survival during follow-up and reviewing patient records.
Results Thirty-nine children, 22 males and 17 females, with a median age of 4 years had positive 131I-mIBG scintigraphy. Intra-abdominal primary lesions and osseous lesions were the most common sites of uptake on 131I-mIBG scan. Of these, 13 had upfront chemotherapy and 26 had surgery followed by chemotherapy. All the patients underwent therapy with 131I-mIBG. Fourteen patients had multiple therapies while the remaining 25 had only one therapy. Eight patients had no follow-up, and 13 had disease relapse. The remaining 18 had regression of disease which was confirmed by follow-up 131I-mIBG scintigraphy and with bone scintigraphy in patients with osseous metastases.
Conclusion 131I-mIBG scintigraphy should be preferred in intermediate and high-risk neuroblastoma to know the extent of the disease and also for patient selection for early therapy with 131I-mIBG. It holds significant utility in the management of metastatic neuroblastoma, facilitating palliative pain relief and tumor size reduction in inoperable or metastatic disease.
Note
The manuscript has been read and approved by all the authors, that the requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Patient Consent
Waiver of consent form obtained as it's a retrospective study.
Publication History
Article published online:
18 July 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Is there a benefit of 131I-MIBG therapy in the treatment of children with stage 4 neuroblastoma?M. Schmidt, Nuklearmedizin, 2006
- Dosimetry for therapeutic treatment of neuroblastoma by 131I-mIBGF. Sudbrock, Nuklearmedizin, 2006
- Image-defined Risk Factors Correlate with Surgical Radicality and Local Recurrence in Patients with NeuroblastomaA. Pohl, Klinische Pädiatrie, 2016
- Image-defined Risk Factors Correlate with Surgical Radicality and Local Recurrence in Patients with NeuroblastomaA. Pohl, VCOT Open, 2016
- Stage 4S Neuroblastoma: What Are the Outcomes? A Systematic Review of Published StudiesArimatias Raitio, European Journal of Pediatric Surgery, 2020
- A Review of Glioblastoma MultiformeGabrielle L. Brown, US Pharmacist, 2010
- High Radiotherapy Dose Improves Outlook for Children With Brain CancerBy staff, US Pharmacist, 2015
- Promising Cortara Data From Brain Cancer StudyUS Pharmacist, 2010
- Chemotherapy Improves Clinical Outcomes in Certain NSCLC PatientsBy staff, US Pharmacist, 2023
- FDA Grants Fast Track Status for EnzastaurinBy staff, US Pharmacist, 2020
Abstract
Introduction Neuroblastoma is the most common extracranial solid tumor in childhood. The data on the treatment experience with 131iodine-meta-iodo-benzyl-guanidine (131I-mIBG) and clinical outcome data are meager from India.
Objectives This article studies the efficacy and treatment outcomes in patients treated with 131I-mIBG in high-risk neuroblastoma.
Materials and Methods The study group consisted of 201 consecutive patients (aged between 1 and 15 years) with biopsy-proven neuroblastoma who underwent 131I-mIBG scans from 2012 to 2022. The majority of these children had a disease that was inoperable or had poor response to chemotherapy. Patients with positive scintigraphy were considered for therapy with 131I-mIBG. The findings were analyzed and correlated with the final diagnosis and outcomes obtained from survival during follow-up and reviewing patient records.
Results Thirty-nine children, 22 males and 17 females, with a median age of 4 years had positive 131I-mIBG scintigraphy. Intra-abdominal primary lesions and osseous lesions were the most common sites of uptake on 131I-mIBG scan. Of these, 13 had upfront chemotherapy and 26 had surgery followed by chemotherapy. All the patients underwent therapy with 131I-mIBG. Fourteen patients had multiple therapies while the remaining 25 had only one therapy. Eight patients had no follow-up, and 13 had disease relapse. The remaining 18 had regression of disease which was confirmed by follow-up 131I-mIBG scintigraphy and with bone scintigraphy in patients with osseous metastases.
Conclusion 131I-mIBG scintigraphy should be preferred in intermediate and high-risk neuroblastoma to know the extent of the disease and also for patient selection for early therapy with 131I-mIBG. It holds significant utility in the management of metastatic neuroblastoma, facilitating palliative pain relief and tumor size reduction in inoperable or metastatic disease.
Keywords
neuroblastoma - 131I-mIBG - 131I-mIBG therapyKey Messages
High-risk neuroblastoma can be treated upfront with 131I-mIBG and used in advanced stages to improve overall survival.
Introduction
Neuroblastoma is the most common extracranial solid tumor of childhood which arises from neural crest cells that form the adrenal medulla and sympathetic ganglia.
About 50%, of patients at diagnosis[1] present with metastasis most commonly to bone or marrow. Important prognostic factors are age at presentation, histological features, tumor ploidy, N-MYC gene amplification, and stage of the disease, which is based on the International Neuroblastoma Staging System (INSS).[2] The worst outcomes are noted in high-risk diseases defined as stage III or IV in children aged more than 18 months at diagnosis as well as those with N-MYC amplification.
The standard of care for high-risk neuroblastoma includes chemotherapy, surgery, myeloablative therapy, and radiation therapy followed by differentiation therapy using cis-retinoic acid. Despite this multimodality treatment, the outcome is poor[3] with overall survival (OS) ranging from 10 to 60%. Immunotherapy using anti-GD2 antibodies has improved outcomes considerably in high-resource countries; however, this modality is currently prohibitively expensive and hence unavailable to the rest of the world. Iodine 131 meta-iodo-benzyl-guanidine (131I-mIBG) is one of the multimodality treatments that is used mainly in advanced stages of neuroblastoma.[4] Treatment of these high-risk neuroblastoma is essential as this may help in planning the management of the disease.
The current study aimed to analyze the treatment outcomes and to look at the feasibility of this form of treatment as a future therapeutic option in this select group of patients from a tertiary care center in India.
Materials and Methods
Inclusion and Exclusion Criteria
Children aged 1 to 15 years, diagnosed to have high-risk neuroblastoma from 2012 to 2022 who had a positive 131I-mIBG scintigraphy were included in this study. Diagnosis of neuroblastoma was confirmed by a biopsy of either the primary tumor or bone marrow (BM) trephine and supported by elevated urine catecholamines. The disease was staged according to the revised INSS and the assessment of response was according to the International Neuroblastoma Response Criteria.[2] 131I-mIBG scintigraphy was done in 201 patients for metastatic workup, among which 168 patients had shown positive uptake. Thirty-nine patients among them who had a positive mIBG scintigraphy during the period of the study received treatment with 131I-mIBG for metastatic or inoperable disease or relapse of the disease after standard care based on decisions by the multidisciplinary tumor board that included pediatric oncologists, surgeons, radiation oncologists, and nuclear medicine physicians.
131I-mIBG Imaging
131I-mIBG which was prepared in-house using carrier-free 131iodine was used[5] to assess for mIBG uptake. A dose of 0.5 mCi was administered intravenously and whole-body planar images were acquired using a gamma camera (Infinia Hawkeye, GE Healthcare, Milwaukee, Wisconsin, United States) at 24, 48, and 72 hours postinjection. Anterior and posterior whole-body images were acquired with a window centered at 364 keV ± 15 and a matrix of 256 × 1024 for 450 s/step in three steps. Single-photon emission computed tomography (CT)/ low-dose CT was acquired for doubtful lesions and anatomical localization.[6]
Posttherapy 131I- mIBG whole-body scintigraphy in anterior and posterior projections was done on the third day after the therapy to look for any lesions not seen on the diagnostic pretherapy scans[7] and also confirm the uptake of mIBG in the target lesions.
131I-mIBG Therapy
Patients were treated in a room specially designed for radioisotope therapy. 131I-mIBG was administered as slow infusion intravenously over 3 to 4 hours with hydration.
Thyroid gland blockade was provided with potassium perchlorate by oral administration from 2 days before therapy to 5 days posttherapy. Blood pressure and heart rate were monitored during the procedure and for 24 hours after treatment. There was no adverse reaction during or shortly after the administration of 131I-mIBG therapy for any of the patients. Dosage was given according to a weight-based regimen with a dose of 37 to 74 MBq/kg (1–2 mCi/kg) to all the patients and patients were discharged once the levels of exposure were < 50 micro-Sv at a 1-m distance which conferred to the Atomic Energy Regulatory Board, India standard guidelines.
Primary Outcome
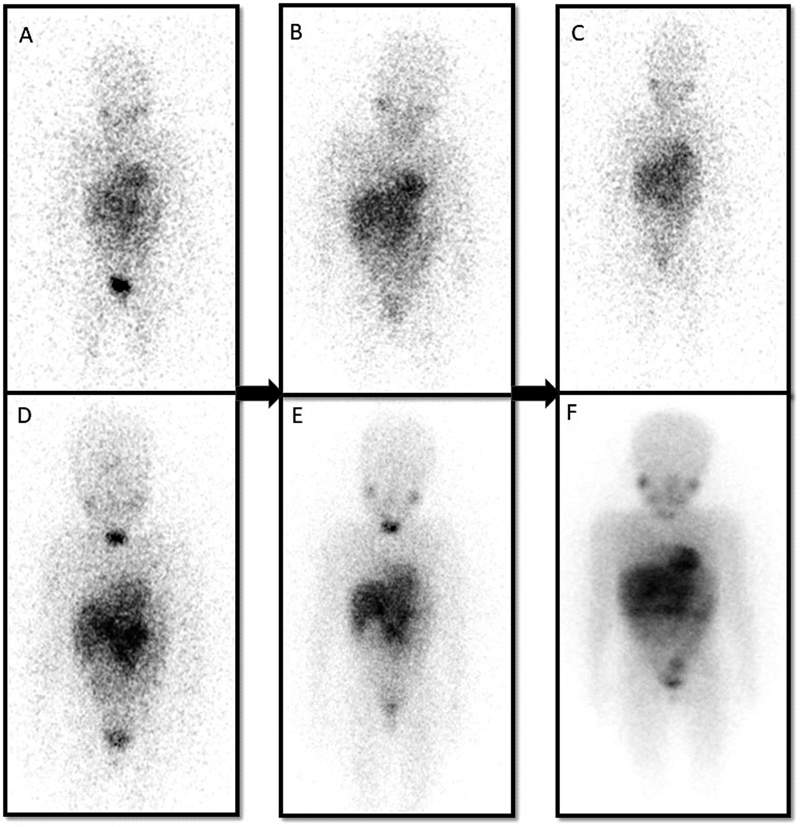
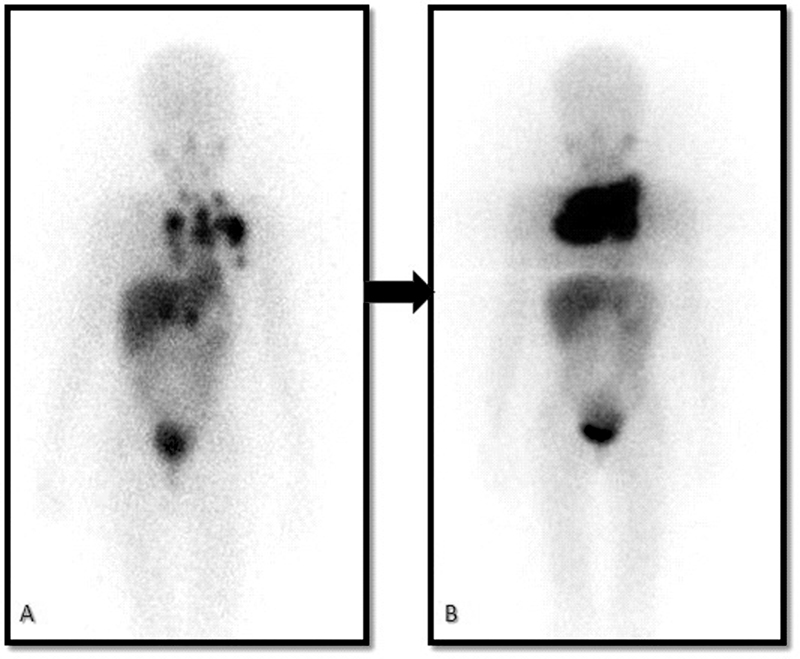
Patients who were treated with 131I-mIBG were followed up after 6 months to look for response evaluation. 131I-mIBG scintigraphy and urinary catecholamine levels were done. Progression of the disease was considered when there was an increase in the intensity of 131I-mIBG uptake or any new lesions noted compared with the posttherapy scan and increase in urinary catecholamine levels ([Fig. 1]). Partial regression of the disease was considered when there was a decrease in the number of lesions or intensity of lesions and decrease in the levels of urinary catecholamine ([Fig. 2]).
| | Fig 1A 6-year-old child with a primary lesion in the mediastinum (A). Progression of the disease as the areas of uptake have increased despite two 131iodine-meta-iodo-benzyl-guanidine (131I-mIBG) therapies on the posttherapy scans (B).|
| Fig 2 A 7-year-old child with a primary abdomen lesion and multiple osseous metastases in the pretherapy scan (A–C). Partial regression is noted as the areas of uptake have significantly reduced after three 131iodine-meta-iodo-benzyl-guanidine (131I-mIBG) therapy as seen in the posttherapy images (D–F).|
Statistical Analysis
Categorical data were summarized using percentages. Numerical data were summarized as the means and standard deviations or medians and ranges. Due to the small sample size multivariable Cox regression for determining independent predictors of survival was not possible.
Ethical Approval Statement
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964, as revised in 2013. Ethics Committee Approval was obtained from the Institutional Ethics Committee vide letter no. IRB Min No. 15495 (RETRO) dated June 28, 2023.
Results
Thirty-nine of 201 children who had a positive 131I-mIBG scan received 131I-mIBG therapy. There were 22 boys and 17 girls with their ages ranging from 1 to 15 years with a median age of 4 years. Six children had stage III disease and all the rest had stage IV neuroblastoma. Twenty-four children received COJEC (cisplatin [C], vincristine [O], carboplatin [J], etoposide [E], and cyclophosphamide [C]) chemotherapy, 7 had carboplatin-etoposide/CADO (cyclophosphamide, doxorubicin, and vincristine), and the rest of the children who had chemotherapy elsewhere prior to coming to our hospital had OPEC (vincristine, prednisolone, etoposide, and chlorambucil)-based chemotherapy. The baseline characteristics of the cohort are shown in [Table 1].
|
Total no. of patients |
39 |
|
Male |
22 |
|
Female |
17 |
|
Age at diagnosis |
|
|
Range |
1–15 |
|
Mean |
5.9 |
|
Median |
4 |
|
Histopathology |
|
|
Neuroblastoma |
35 |
|
Ganglioneuroblastoma |
4 |
|
Immunohistochemistry |
|
|
Synaptophysin, chromogranin, and NSE |
35 of 39 (4 were operated elsewhere) |
|
Stage of the disease |
|
|
Stage 3 |
7 |
|
Stage 4 |
32 |
|
Presentation |
|
|
Inoperable primary |
20 |
|
Skeletal metastasis |
13 |
|
Primary with skeletal metastasis |
3 |
|
Extraosseous metastasis |
3 |
|
Treatments prior to 131I-mIBG therapy |
|
|
Chemotherapy followed by |
|
|
Debulking surgery |
28 |
|
Chemotherapy |
11 |
|
131I-mIBG therapies |
57 |
|
1 dose |
25 |
|
2 doses |
10 |
|
3 doses |
4 |
|
131I-mIBG activity (mCi) |
37–74 MBq/kg body weight |
|
1–2 mCi/kg body weight |
|
|
Follow-up |
|
|
Period |
12–60 mo |
|
Mean |
25 mo |
|
Results based on posttherapy 131I-mIBG scan |
|
|
Regression |
18 |
|
Progression |
12 |
|
Lost to follow-up |
9 |
|
Post 131I-mIBG treatment in progressive disease |
|
|
Chemotherapy |
7 |
|
Radiation therapy |
1 |
|
Supportive/palliative treatment |
4 |
References
- DuBois SG, Kalika Y, Lukens JN. et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol 1999; 21 (03) 181-189
- Orr KE, McHugh K. The new International Neuroblastoma Response Criteria. Pediatr Radiol 2019; 49 (11) 1433-1440
- Yu AL, Gilman AL, Ozkaynak MF. et al; Children's Oncology Group. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010; 363 (14) 1324-1334
- Garaventa A, Bellagamba O, Lo Piccolo MS. et al. 131I-metaiodobenzylguanidine (131I-MIBG) therapy for residual neuroblastoma: a mono-institutional experience with 43 patients. Br J Cancer 1999; 81 (08) 1378-1384
- Oommen R, Shanthly N, Subramani N. et al. In-house preparation of iodine -131 metaiodo benzyl guanidine for scintigraphy of neuroendocrine tumors. Fourteen years experience in South India. Hell J Nucl Med 2007; 10 (03) 164-166
- Verma P, Chanadana, Hephzibah J, Shanthly N, Oommen R. Iodine-131MIBG SPECT/CT in neuroendocrine tumours: an institutional experience. Indian J Nucl Med 2012; 27 (04) 246-248
- Wakabayashi H, Kayano D, Inaki A. et al. Diagnostic use of post-therapy 131I-meta-iodobenzylguanidine scintigraphy in consolidation therapy for children with high-risk neuroblastoma. Diagnostics (Basel) 2020; 10 (09) 663
- Taggart DR, Han MM, Quach A. et al. Comparison of iodine-123 metaiodobenzylguanidine (MIBG) scan and [18F]fluorodeoxyglucose positron emission tomography to evaluate response after iodine-131 MIBG therapy for relapsed neuroblastoma. J Clin Oncol 2009; 27 (32) 5343-5349
- Bansal D, Marwaha RK, Trehan A, Rao KLN, Gupta V. Profile and outcome of neuroblastoma with convertional chemotherapy in children older than one year: a 15-years experience. Indian Pediatr 2008; 45 (02) 135-139
- DuBois SG, Mody R, Naranjo A. et al. MIBG avidity correlates with clinical features, tumor biology, and outcomes in neuroblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer 2017; 64 (11) 10
- Schmidt M, Simon T, Hero B. et al. Is there a benefit of 131 I-MIBG therapy in the treatment of children with stage 4 neuroblastoma? A retrospective evaluation of The German Neuroblastoma Trial NB97 and implications for The German Neuroblastoma Trial NB2004. Nucl Med (Stuttg) 2006; 45 (04) 145-151 , quiz N39–N40
- Hoefnagel CA, De Kraker J, Valdés Olmos RA, Voûte PA. 131I-MIBG as a first-line treatment in high-risk neuroblastoma patients. Nucl Med Commun 1994; 15 (09) 712-717
- Howard JP, Maris JM, Kersun LS. et al. Tumor response and toxicity with multiple infusions of high dose 131I-MIBG for refractory neuroblastoma. Pediatr Blood Cancer 2005; 44 (03) 232-239
- Anongpornjossakul Y, Sriwatcharin W, Thamnirat K. et al. Iodine-131 metaiodobenzylguanidine (131I-mIBG) treatment in relapsed/refractory neuroblastoma. Nucl Med Commun 2020; 41 (04) 336-343
- Sisson JC, Shapiro B, Hutchinson RJ. et al. Predictors of toxicity in treating patients with neuroblastoma by radiolabeled metaiodobenzylguanidine. Eur J Nucl Med 1994; 21 (01) 46-52
- Clement SC, van Rijn RR, van Eck-Smit BLF. et al. Long-term efficacy of current thyroid prophylaxis and future perspectives on thyroid protection during 131I-metaiodobenzylguanidine treatment in children with neuroblastoma. Eur J Nucl Med Mol Imaging 2015; 42 (05) 706-715
- Radhakrishnan V, Raja A, Dhanushkodi M, Ganesan TS, Selvaluxmy G, Sagar TG. Real world experience of treating neuroblastoma: experience from a tertiary cancer centre in India. Indian J Pediatr 2019; 86 (05) 417-426
- de Kraker J, Hoefnagel KA, Verschuur AC, van Eck B, van Santen HM, Caron HN. Iodine-131-metaiodobenzylguanidine as initial induction therapy in stage 4 neuroblastoma patients over 1 year of age. Eur J Cancer 2008; 44 (04) 551-556
- Johnson K, McGlynn B, Saggio J. et al. Safety and efficacy of tandem 131I-metaiodobenzylguanidine infusions in relapsed/refractory neuroblastoma. Pediatr Blood Cancer 2011; 57 (07) 1124-1129
-
Samim A,
Blom T,
Poot AJ.
et al.
[18F]mFBG PET-CT for detection and localisation of neuroblastoma: a prospective pilot study. Eur J Nucl Med Mol Imaging 2023; 50 (04) 1146-1157
Address for correspondence
Julie Hephzibah, MD, DNBDepartment of Nuclear Medicine, Christian Medical CollegeVellore 632004, Tamil NaduIndiaEmail: drjulsan@cmcvellore.ac.inPublication History
Article published online:
18 July 2024© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, IndiaWe recommend- Is there a benefit of 131I-MIBG therapy in the treatment of children with stage 4 neuroblastoma?M. Schmidt, Nuklearmedizin, 2006
- Dosimetry for therapeutic treatment of neuroblastoma by 131I-mIBGF. Sudbrock, Nuklearmedizin, 2006
- Image-defined Risk Factors Correlate with Surgical Radicality and Local Recurrence in Patients with NeuroblastomaA. Pohl, Klinische Pädiatrie, 2016
- Image-defined Risk Factors Correlate with Surgical Radicality and Local Recurrence in Patients with NeuroblastomaA. Pohl, VCOT Open, 2016
- Stage 4S Neuroblastoma: What Are the Outcomes? A Systematic Review of Published StudiesArimatias Raitio, European Journal of Pediatric Surgery, 2020
- DO THE RADIOLOGICAL CRITERIA WITH THE USE OF RISK FACTORS IMPACT THE FORECASTING OF ABDOMINAL NEUROBLASTIC TUMOR RESECTION IN CHILDREN?Ana Cláudia Soares PENAZZI, ABCD Arq Bras Cir Dig, 2017
- Pediatric Neuroblastoma Diagnosis Using Urinary Catecholamine MetabolitesNancy Ogbonna, Global Rph, 2023
- Remission of Renal Cell Carcinoma With Brain Metastases: A Case StudyArmela Escalona, Global Rph, 2022
- LIVER TRANSPLANT FOR METASTATIC NEUROENDOCRINE TUMORS A SINGLE-CENTER REPORTRaquel Lima Sampaio, ABCD Arq Bras Cir Dig, 2023
- TWELVE YEARS OF EXPERIENCE USING CHOLECYSTOJEJUNAL BY-PASS FOR PALLIATIVE TREATMENT OF ADVANCED PANCREATIC CANCERMarcos Belotto de OLIVEIRA, ABCD Arq Bras Cir Dig, 2017
- Is there a benefit of 131I-MIBG therapy in the treatment of children with stage 4 neuroblastoma?

| | Fig 1A 6-year-old child with a primary lesion in the mediastinum (A). Progression of the disease as the areas of uptake have increased despite two 131iodine-meta-iodo-benzyl-guanidine (131I-mIBG) therapies on the posttherapy scans (B).|

| Fig 2 A 7-year-old child with a primary abdomen lesion and multiple osseous metastases in the pretherapy scan (A–C). Partial regression is noted as the areas of uptake have significantly reduced after three 131iodine-meta-iodo-benzyl-guanidine (131I-mIBG) therapy as seen in the posttherapy images (D–F).|
References
- DuBois SG, Kalika Y, Lukens JN. et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol 1999; 21 (03) 181-189
- Orr KE, McHugh K. The new International Neuroblastoma Response Criteria. Pediatr Radiol 2019; 49 (11) 1433-1440
- Yu AL, Gilman AL, Ozkaynak MF. et al; Children's Oncology Group. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010; 363 (14) 1324-1334
- Garaventa A, Bellagamba O, Lo Piccolo MS. et al. 131I-metaiodobenzylguanidine (131I-MIBG) therapy for residual neuroblastoma: a mono-institutional experience with 43 patients. Br J Cancer 1999; 81 (08) 1378-1384
- Oommen R, Shanthly N, Subramani N. et al. In-house preparation of iodine -131 metaiodo benzyl guanidine for scintigraphy of neuroendocrine tumors. Fourteen years experience in South India. Hell J Nucl Med 2007; 10 (03) 164-166
- Verma P, Chanadana, Hephzibah J, Shanthly N, Oommen R. Iodine-131MIBG SPECT/CT in neuroendocrine tumours: an institutional experience. Indian J Nucl Med 2012; 27 (04) 246-248
- Wakabayashi H, Kayano D, Inaki A. et al. Diagnostic use of post-therapy 131I-meta-iodobenzylguanidine scintigraphy in consolidation therapy for children with high-risk neuroblastoma. Diagnostics (Basel) 2020; 10 (09) 663
- Taggart DR, Han MM, Quach A. et al. Comparison of iodine-123 metaiodobenzylguanidine (MIBG) scan and [18F]fluorodeoxyglucose positron emission tomography to evaluate response after iodine-131 MIBG therapy for relapsed neuroblastoma. J Clin Oncol 2009; 27 (32) 5343-5349
- Bansal D, Marwaha RK, Trehan A, Rao KLN, Gupta V. Profile and outcome of neuroblastoma with convertional chemotherapy in children older than one year: a 15-years experience. Indian Pediatr 2008; 45 (02) 135-139
- DuBois SG, Mody R, Naranjo A. et al. MIBG avidity correlates with clinical features, tumor biology, and outcomes in neuroblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer 2017; 64 (11) 10
- Schmidt M, Simon T, Hero B. et al. Is there a benefit of 131 I-MIBG therapy in the treatment of children with stage 4 neuroblastoma? A retrospective evaluation of The German Neuroblastoma Trial NB97 and implications for The German Neuroblastoma Trial NB2004. Nucl Med (Stuttg) 2006; 45 (04) 145-151 , quiz N39–N40
- Hoefnagel CA, De Kraker J, Valdés Olmos RA, Voûte PA. 131I-MIBG as a first-line treatment in high-risk neuroblastoma patients. Nucl Med Commun 1994; 15 (09) 712-717
- Howard JP, Maris JM, Kersun LS. et al. Tumor response and toxicity with multiple infusions of high dose 131I-MIBG for refractory neuroblastoma. Pediatr Blood Cancer 2005; 44 (03) 232-239
- Anongpornjossakul Y, Sriwatcharin W, Thamnirat K. et al. Iodine-131 metaiodobenzylguanidine (131I-mIBG) treatment in relapsed/refractory neuroblastoma. Nucl Med Commun 2020; 41 (04) 336-343
- Sisson JC, Shapiro B, Hutchinson RJ. et al. Predictors of toxicity in treating patients with neuroblastoma by radiolabeled metaiodobenzylguanidine. Eur J Nucl Med 1994; 21 (01) 46-52
- Clement SC, van Rijn RR, van Eck-Smit BLF. et al. Long-term efficacy of current thyroid prophylaxis and future perspectives on thyroid protection during 131I-metaiodobenzylguanidine treatment in children with neuroblastoma. Eur J Nucl Med Mol Imaging 2015; 42 (05) 706-715
- Radhakrishnan V, Raja A, Dhanushkodi M, Ganesan TS, Selvaluxmy G, Sagar TG. Real world experience of treating neuroblastoma: experience from a tertiary cancer centre in India. Indian J Pediatr 2019; 86 (05) 417-426
- de Kraker J, Hoefnagel KA, Verschuur AC, van Eck B, van Santen HM, Caron HN. Iodine-131-metaiodobenzylguanidine as initial induction therapy in stage 4 neuroblastoma patients over 1 year of age. Eur J Cancer 2008; 44 (04) 551-556
- Johnson K, McGlynn B, Saggio J. et al. Safety and efficacy of tandem 131I-metaiodobenzylguanidine infusions in relapsed/refractory neuroblastoma. Pediatr Blood Cancer 2011; 57 (07) 1124-1129
- Samim A, Blom T, Poot AJ. et al. [18F]mFBG PET-CT for detection and localisation of neuroblastoma: a prospective pilot study. Eur J Nucl Med Mol Imaging 2023; 50 (04) 1146-1157


 PDF
PDF  Views
Views  Share
Share

